400-6699-117转1000
咨询列表
广州市华粤行仪器有限公司
您好,欢迎您查看分析测试百科网,请问有什么帮助您的?







诚信认证:
工商注册信息已核实! 扫一扫即可访问手机版展台
扫一扫即可访问手机版展台
| 参考报价: | 面议 | 型号: | NEPA21 |
| 品牌: | Nepa Gene | 产地: | 日本 |
| 关注度: | 441 | 信息完整度: | |
| 样本: | 典型用户: | 8个 |
400-6699-117转1000
NEPA21高效基因转染系统
---适用于体外(In Vitro)和活体(In Vivo)
NEPA GENE公司专业研发、生产细胞电转染仪及电融合仪等,其生产CUY21系列电转染仪在研究领域中久负盛名,已被数百篇文献引用,其中不乏高水平杂志的文章,如Nature、Cell、PNAS、Genes & Dvelopment等。
2011年,NEPA GENE公司推出新一代全能型NEPA21高效基因转染系统。与传统的电转仪相比,NEPA21采用全新设计的电转程序,特别适用于难转染细胞、离体组织或动物活体的转染。配合独有的电压衰减(Voltage Decay)设计,NEPA21可在获得高转染效率的同时,提高细胞存活率。
NEPA21高效基因转染系统应用范围十分广泛:
► 对真核细胞进行快速、高效、高存活率的基因转染,特别针对难转染的细胞,如原代细胞、神经细胞、干细胞、悬浮细胞等提供优化的实验方案
► 对贴壁状态的细胞直接进行转染,无需经过细胞消化、悬浮的过程,增加了细胞存活率和实验的简便性
► 对离体的组织或器官进行转染(Ex Vivo Transfection)
► 进行活体动物的基因转染(In Vivo Transfection)
NEPA21高效基因转染系统的优点: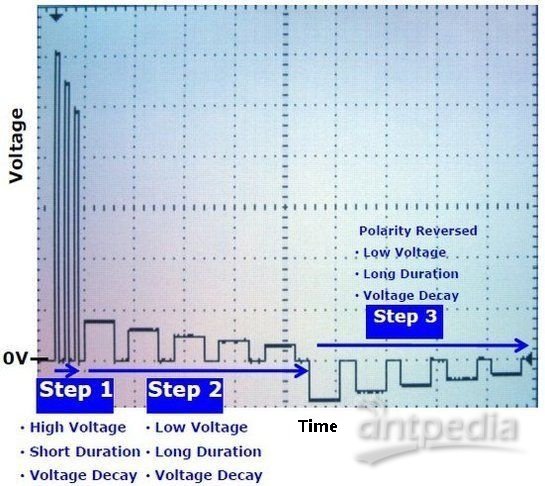 • 全新设计的电转程序
• 全新设计的电转程序
• 特别适用于难转染细胞、离体组织或动物活体的转染
• 适用于DNA和RNA(如siRNA等)转染
• 高转染效率、高细胞存活率
• 电转程序各项参数可见、可调,适用性广
• 不需要特殊的转染试剂盒辅助,运维成本低
主要特点:
全新设计的电转程序,
特别适用于难转染细胞、离体组织或动物活体的转染,
高转染效率、高细胞存活率
电转程序各项参数可见、可调,适用性广
不需要特殊的转染试剂盒辅助,运维成本低
应用范围:
♦ 悬浮转染
适用范围:原代细胞、干细胞、以及各种难转染细胞(如免疫细胞、血液细胞等)
♦ 贴壁转染
适用范围:可以直接对贴壁细胞进行转染,省去了细胞消化、再贴壁的步骤(对提高某些种类细胞的存活率十分重要)!
♦ 离体组织转染
适用范围:组织切片、脑切片、器官、胚胎等
♦ 活体转染
适用范围:大脑、视网膜、肌肉、皮肤、肝脏、肾脏、睾丸等
部分参考文献:
细胞转染仪(NEPA21、CUY21系列):
Barnabe-Heider et al. Genetic manipulation of adult mouse neurogenic niches by in vivo electroporation. Nature Methods, 2008 Feb;5(2):189-96.
Shibata MA, et al. Combination therapy with short interfering RNA vectors against VEGF-C and VEGF-A suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther. 2008 Dec;15(12):776-86.
Limura T and Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature, 2006 Aug 3;442(7102):568-71.
Sanada K and Tsai LH. G Protein betagamma Subunits and AGS3 Control Spindle Orientation and Asymmetric Cell Fate of Cerebral Cortical Progenitors. Cell, 2005 Jul 15;122(1):119-31
Ladher RK et al. FGF8 initiates inner ear induction in chick and mouse. Genes and Development, 2005 Mar 1;19(5):603-13.
更多文献或产品信息,请浏览英文网址:或本公司网址
想了解活体转染操作技术?世界上首位报道用电转染法进行小鼠胚胎大脑活体转染的科学家(Neuroscience. 2001;103(4):865-72),庆应大学Keio University的Dr H Tabata亲自操刀为您演示的胚胎活体转染操作方法。请点击观看视频:
引用文献:
NEPA21
[1].Kobayashi, Y., et al., ERK1/2 mediates unbalanced growth leading to senescence induced by excess thymidine in human cells. Biochemical and Biophysical Research Communications, 2012. 425(4): p. 897-901.
[2].Kusuzawa, S., et al., Leucine-rich glioma inactivated 1 (Lgi1), an epilepsy-related secreted protein, has a nuclear localization signal and localizes to both the cytoplasm and the nucleus of the caudal ganglionic eminence neurons. European Journal of Neuroscience, 2012. 36(3): p. 2284-2292.
[3].Hattori, Y., et al., A selective estrogen receptor modulator inhibits tumor necrosis factor-α-induced apoptosis through the ERK1/2 signaling pathway in human chondrocytes. Biochemical and Biophysical Research Communications, 2012. 421(3): p. 418-424.
[4].Aoyagi, K., et al., Acute Inhibition of PI3K-PDK1-Akt Pathway Potentiates Insulin Secretion through Upregulation of Newcomer Granule Fusions in Pancreatic β-Cells. PLoS ONE, 2012. 7(10): p. e47381
[5].Choijookhuu, N., et al., Estrogen-dependent regulation of sodium/hydrogen exchanger-3 (NHE3) expression via estrogen receptor β in proximal colon of pregnant mice. Histochemistry and Cell Biology, 2012. 137(5): p. 575-587.
[6].Shirakabe, K., et al., VIP36 Protein Is a Target of Ectodomain Shedding and Regulates Phagocytosis in Macrophage Raw 264.7 Cells. Journal of Biological Chemistry, 2011. 286(50): p. 43154 -43163.
CUY21
[1].Katsumura, K.R., S. Maruo and K. Takada, EBV lytic infection enhances transformation of B-lymphocytes infected with EBV in the presence of T-lymphocytes. Journal of Medical Virology, 2012. 84(3): p. 504-510.
[2].Usui, N., et al., Role of motoneuron-derived neurotrophin 3 in survival and axonal projection of sensory neurons during neural circuit formation. Development, 2012. 139(6): p. 1125 -1132.
[3].Yamaguchi, S., et al., Molecular function of microtubule-associated protein 2 for filial imprinting in domestic chicks (Gallus gallus domesticus). Neuroscience Research, 2011. 69(1): p. 32-40.
[4].Iida, A., et al., Dicer Plays Essential Roles for Retinal Development by Regulation of Survival and Differentiation. Investigative Ophthalmology & Visual Science, 2011. 52(6): p. 3008 -3017.
[5].Tachibana, Y., et al., Design and characterization of a polymeric MRI contrast agent based on PVA for in vivo living-cell tracking. Contrast Media & Molecular Imaging, 2010. 5(6): p. 309-317.
[6].Li, Q., et al., Production of human lysozyme-transgenic cloned porcine embryos by somatic nuclear transfer. Progress in Natural Science, 2009. 19(6): p. 699-704.
[7].Furne, C., et al., Netrin-1 is a survival factor during commissural neuron navigation. Proceedings of the National Academy of Sciences, 2008. 105(38): p. 14465 -14470.
[8].Kiyama, S., et al., Reduction of fibrosis in a rat model of non-alcoholic steatohepatitis cirrhosis by human HGF gene transfection using electroporation. Journal of Gastroenterology and Hepatology, 2008. 23(8pt2): p. e471-e476.
[9].ZHANG, K., et al., Effects of Ghrelin on In Vitro Development of Porcine In Vitro Fertilized and Parthenogenetic Embryos. Journal of Reproduction and Development, 2007. 53(3): p. 647-653.
[10].Luo, J., M.J. Ju and C. Redies, Regionalized cadherin-7 expression by radial glia is regulated by Shh and Pax7 during chicken spinal cord development. Neuroscience, 2006. 142(4): p. 1133-1143.
[11].Smith, T.G., et al., Negative feedback predominates over cross-regulation to control ERK MAPK activity in response to FGF signalling in embryos. FEBS Letters, 2006. 580(17): p. 4242-4245.
[12].Blackmore, M. and P.C. Letourneau, L1, β1 integrin, and cadherins mediate axonal regeneration in the embryonic spinal cord. Journal of Neurobiology, 2006. 66(14): p. 1564-1583.
[13].Tada, M., et al., Use of local electroporation enhances methotrexate effects with minimum dose in adjuvant-induced arthritis. Arthritis & Rheumatism, 2005. 52(2): p. 637-641.
[14].Matsunaga, E., et al., RGM and its receptor neogenin regulate neuronal survival. 2004. 6(8): p. 749-755.
[15].Matsuda, T. and C.L. Cepko, Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proceedings of the National Academy of Sciences, 2004. 101(1): p. 16 -22.
[16].Ma, J., et al., Local Actions of Endogenous Angiotensin II in Injured Glomeruli. Journal of the American Society of Nephrology, 2004. 15(5): p. 1268 -1276.
[17].Yoshida, M., et al., Gene Therapy for Central Diabetes Insipidus: Effective Antidiuresis by Muscle-Targeted Gene Transfer. Endocrinology, 2004. 145(1): p. 261 -268.
[18].Yomogida, K., et al., Dramatic Expansion of Germinal Stem Cells by Ectopically Expressed Human Glial Cell Line-Derived Neurotrophic Factor in Mouse Sertoli Cells. Biology of Reproduction, 2003. 69(4): p. 1303 -1307.
[19].Jin, Z., et al., Irx4-mediated regulation of Slit1 expression contributes to the definition of early axonal paths inside the retina. Development, 2003. 130(6): p. 1037 -1048.
[20].Sasagawa, S., et al., Improved mRNA electroporation method for Xenopus neurula embryos. genesis, 2002. 33(2): p. 81-85.
[21].Xue, F., et al., Attenuated acute liver injury in mice by naked hepatocyte growth factor gene transfer into skeletal muscle with electroporation. Gut, 2002. 50(4): p. 558 -562.
[22].Sasagawa, S., et al., Axes establishment during eye morphogenesis in Xenopus by coordinate and antagonistic actions of BMP4, Shh, and RA. genesis, 2002. 33(2): p. 86-96.
更多产品信息,请浏览NEPAGENE英文网址:http://www.nepagene.jp/E/Eindex.htm
世界上首位报道用电转染法进行小鼠胚胎大脑活体转染(Neuroscience.2001;103(4):865-72)的科学家庆应大学Keio University的Dr H Tabata亲自动手演示胚胎活体转染的操作方法,请点击观看视频:http://video.sina.com.cn/v/b/63556016-2484835144.html
NEPA21 高效基因转染系统,电转仪信息由广州市华粤行仪器有限公司为您提供,如您想了解更多关于NEPA21 高效基因转染系统,电转仪报价、型号、参数等信息,欢迎来电或留言咨询。
注:该产品未在中华人民共和国食品药品监督管理部门申请医疗器械注册和备案,不可用于临床诊断或治疗等相关用途