Gamry电化学工作站:拉曼光谱电化学基础
Gamry电化学工作站:拉曼光谱电化学基础
Purpose of This Note
This application note discusses Raman spectroscopy and its combination with electrochemical techniques.
The theory of Raman spectroscopy and the effect of light on matter are explained. Further, the general setup for Raman spectroscopy is shown including its extension to spectroelectrochemical measurements. Gamry’s measurement software and data evaluation are explained based on spectroelectrochemical experiments.
Introduction
Raman spectroscopy is a widely used spectroscopic method. Highly specific spectra of materials can be obtained which can be compared and identified by using spectral databases. Similar to IR-spectroscopy, fundamental vibrations of molecules are examined which is important for a complete understanding of chemical reactions.
However, in contrast to IR-spectroscopy, no absorption effects are observed but scattering of light. As water is a strong absorber, Raman spectroscopy is the method of choice for studying aqueous solutions compared to IR-spectroscopy. This makes it suitable for biological and medical research,e.g. analysis of the impact of drugs on biological cells.
Raman spectra can be acquired very fast. Hence it is used for a large variety of in-situ analyses. Further, it is in general a non-destructive technique depending on the intensity of the laser and duration of an experiment.
The experimental setup is simple as no sample preparation is necessary. Solid or liquid samples can be used as they are received. Experiments can be performed either inside or outside of a measurement cell through glass or plastic.
Raman Spectroscopy
Theory
When light is focused on matter, both interact in different ways with each other. Light can be absorbed, scattered, transmitted, or reflected amongst other effects which would go beyond the scope of this discussion.
In 1828, the Indian physicist Sir C. V. Raman performed a series of measurements where he focused sunlight on a liquid probe (see Figure 1).
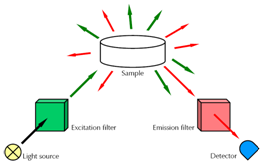
Figure 1 – Simplified setup of a Raman experiment.
He used a monochromatic filter (excitation filter) which let only light with a specific wavelength reach the probe. The measured scattered light showed a broader spectrum with additional wavelengths. A second filter (emission filter) behind the probe allowed blocking the incident wavelength. The observed residual scattered light could now be clearly distinguished from the incident light.
Light scattering
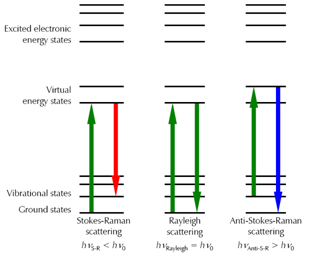
The observations which Sir Raman made can be explained by the fact that photons which are not absorbed by the probe will be scattered.
In UV-Vis absorption spectroscopy, electrons in the ground state are excited to a so-called excited electronic state. For this, the photon energy (depending on the wavelength) has to match the difference in the energy states. As a result, those absorbed wavelengths cannot be found in the transmitting light.
When light is scattered, electrons are also excited from their ground state. However, the photon energy is does not have to be resonant. Molecules can be excited to a virtual energy state, see Figure 2.