国际纯水标准
International Organization for Standardization specification for water for laboratory use ISO 3696: 1995
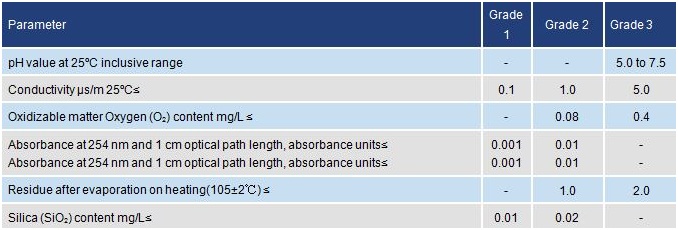
This standard covers three grades of water as follows:
Grade 1
Essentially
free from dissolved or colloidal ionic and organic contaminants. It is
suitable for the most stringent analytical requirements including those
of high performance liquid chromatography (HPLC). It should be produced
by further treatment of grade 2 water for example by reverse osmosis or
ion exchange followed by filtration through a membrane filter of pore
size 0.2µm to remove particle matter or re-distillation from a fused
silica apparatus.
Grade 2
Very
low inorganic, organic or colloidal contaminants and suitable for
sensitive analytical purposes including atomic absorption spectrometry
(AAS) and the determination of constituents in trace quantities. Can be
produced by multiple distillation, ion exchange or reverse osmosis
followed by distillation.
Grade 3
Suitable
for most laboratory wet chemistry work and preparation of reagent
solutions. Can be produced by single distillation, by ion exchange, or
by reverse osmosis. Unless otherwise specified, it should be used for
ordinary analytical work.
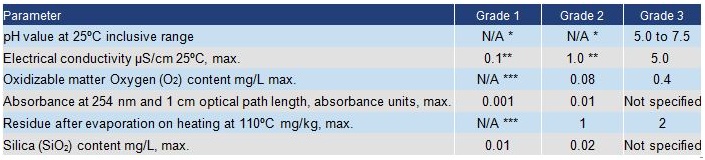
American Society for Testing and Materials (ASTM) D1193-2006 Standard specification for Reagent Grade Water
This specification covers requirements for water suitable for use in methods of chemical analysis and physical testing, the choice of one of the various grades being designated by the method or the investigator.
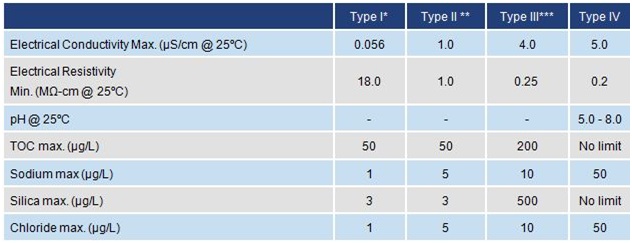
Key:
*Requires the use of 0.2µm membrane filter
** Prepared by distillation
*** Requires the use of a 0.45µm membrane filter
When bacterial levels need to be controlled, reagent grade types should be further classified as follows:
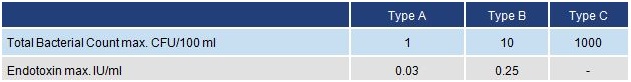
Clinical and Laboratory Standards Institute (CLSI) (C3-A4)
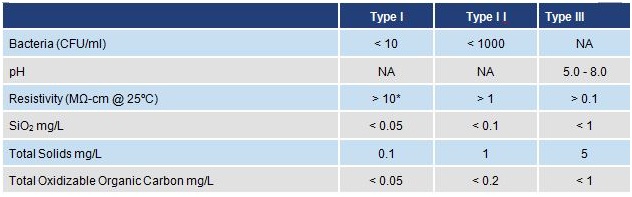
Type I water must be free of particulate matter larger than 0.2µm
* Resistivity of Type I must be measured in-line
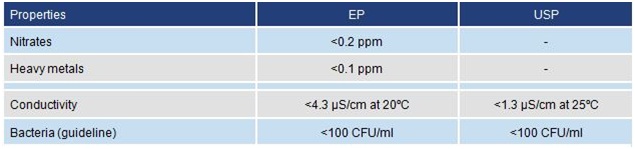
Pharmacopoeia standards
Separate
pharmacopoeia are produced by a number of authorities, notably in the
USA and Europe. Each specifies materials, including water, to be used in
medical work. The standards for purified water are similar in each
case. Extra criteria are set for water required for sterile
applications. The standards for purified water given in the European
Pharmacopoeia (EP) and in the US Pharmacopoeia (USP) are summarized
below. Water for injection has stringent bacterial/pyrogen criteria and
methods of preparation are specified.
Pharmacopoeia requirements for purity of "purified water"
Water for analytical laboratory use-Specification and test methods (China GB/T 6682-2008)