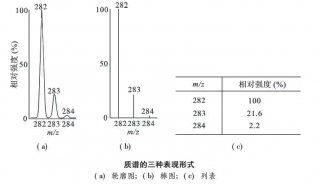
JIP-test荧光数据及其它生理生态数据主成分综合分析...-3
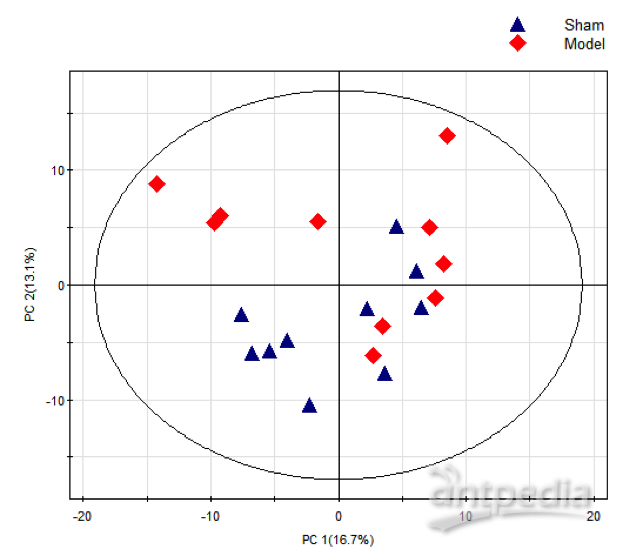
通过PCA技术分析发现,所选的JIP-test参数形成了三个很好分离的簇。其中两个位于PC1(Cluster 1&2)上,一个(Cluster 3)位于PC2上。每一组参数描述了不同的生理过程:光能捕获和光化学阶段(Cluster 1)、耗散(Cluster 2)和热阶段(Cluster 3)。基于PCA分析,该研究认为样品的整体光合性能可以用PITOT来表示,或者用Fv/Fm和ΔVIP共同来表示。

图3. 所选JIP-test参数的主成分分析
在大多数情况下,植物的光合性能可用Fv/Fm和ΔVIP来描述。经过验证,Fv/Fm和ΔVIP能够有效地代表各种调查(野外和实验室)、气候和时间跨度、植物物种和功能群(针叶树和阔叶树物种、草本植物)中样本的变异性。因此,可用于探索性调查,以筛选大样本植物的光合性能,以及它们对不同生态条件的适应性。
在林业或生态学调查方面,以JIP-test为代表的植被叶绿素荧光特性的大规模野外调查对验证无人机或卫星遥感观测结果具有重要意义,遥感观测数据和野外实地调查数据之间的衔接将是今后生态学研究的一个重要领域(Bussotti F, 2020)!
总述
以上实例说明,PCA分析与JIP-test结合应用越来越广泛,大大提高了数据分析效率,能够快速判断实验处理后的主要变化,并分析主要影响因素,从而对实验材料进行预判。近年来,PCA在植物科学研究中的应用呈上升趋势,相信科研工作者们会开发出更多更好的应用方向。
如何实现对叶绿素a荧光数据(JIP-test参数)、其它生理参数和基因、蛋白等分子数据组成的大数据库进行PCA分析?
通常我们可以使用学术界常用的商用数据分析软件进行PCA分析,如SPSS Statistics(IBM Corp)、Statistica(StatSoft Inc. 2011)和SAS(SAS Enterprise Miner; SAS Institute, Cary, NC)等。
在全球互联网化的大趋势下,也涌现出一批使用体验更佳、分析更加智能化的在线数据分析工具,如SPSSAU(QingSi Technology Ltd 2016-2020)、ClustVis(Metsalu, Tauno et al. 2015)等。
此外以R语言和Python为代表的计算机程序设计语言可以实现对大数据的快速智能处理、计算和制图,使用R语言和Python对JIP-test荧光数据进行PCA数据分析也已有非常成熟的语言包进行应用。
本文参考文献
[1] Baeten,L., Verheyen, K., et al. A novel comparative research platform designed to determinethe functional significance of tree species diversity in European forests. Perspect.Plant Ecol. Evol. Syst. 2013, 15 (5), 281–291.
[2] Bussotti F, Gerosa G, Digrado A, et al.Selection of chlorophyll fluorescence parameters as indicators ofphotosynthetic efficiency in large scale plant ecological studies[J].Ecological Indicators, 2020, 108: 105686.
[3] Goltsev V, Zaharieva I, Chernev P, et al.Drought-induced modifications of photosynthetic electron transport in intactleaves: analysis and use of neural networks as a tool for a rapid non-invasiveestimation[J]. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 2012,1817(8): 1490-1498.
[4] Kalaji H M, Bąba W, Gediga K, et al.Chlorophyll fluorescence as a tool for nutrient status identification inrapeseed plants[J]. Photosynthesis research, 2018, 136(3): 329-343.
[5] Kalaji H M, Schansker G, Brestic M, et al.Frequently asked questions about chlorophyll fluorescence, the sequel[J].Photosynthesis Research, 2017, 132(1): 13-66.
[6] Metsalu, Tauno and Vilo, Jaak. Clustvis: a webtool for visualizing clustering of multivariate data using Principal ComponentAnalysis and heatmap. Nucleic Acids Research, 43(W1): W566–W570, 2015.
[7] Pontes. D, Ontes, M.,Rodriguez, R. and Santiago,E.F. Letter to The Editor. The energy flux theorycelebrates 40 years: toward asystems biology concept? Photosynthetica,2019, vol. 57, iss. 2, p.521-522.
[8] Tsimilli-michael M. Revisiting JIP-test: Aneducative review on concepts, assumptions, approximations, definitions andterminology[J].Photosynthetica,2019,57 (SI): 90-107.
[9] Verheyen, K., Vanhellemont, M., et al. 2016.Contributions of a global network of tree diversity experiments to sustainableforest plantations. Ambio 45, 29–41.
应用PCA分析JIP-test荧光数据文献节选
[1] DimitrovaS, Paunov M, Pavlova B, et al. Special issue in honour of Prof. Reto J.Strasser–Photosynthetic efficiency of two Platanus orientalis L. ecotypesexposed to moderately high temperature-JIP-test analysis[J]. Photosynthetica,2020, 58(SPECIAL ISSUE): 657-670.
[2] BussottiF, Gerosa G, Digrado A, et al. Selection of chlorophyll fluorescence parametersas indicators of photosynthetic efficiency in large scale plant ecologicalstudies[J]. Ecological Indicators, 2020, 108: 105686.
[3] MihaljevićI, Lepeduš H, Šimić D, et al. Photochemical efficiency of photosystem II in twoapple cultivars affected by elevated temperature and excess light in vivo[J].South African Journal of Botany, 2020, 130: 316-326.
[4] Franić M,Jambrović A, Šimić D, et al. Photosynthetic properties of maize hybrids underdifferent environmental conditions probed by the chlorophyll a fluorescence[J].Maydica, 2020, 64(3): 9.
[5] Plich J,Boguszewska-Mańkowska D, Marczewski W. Relations Between PhotosyntheticParameters and Drought-Induced Tuber Yield Decrease in Katahdin-Derived PotatoCultivars[J]. Potato Research, 2020: 1-15.
[6] Vuletic M,Španic V. Special issue in honour of Prof. Reto J. Strasser–Characterization ofphotosynthetic performance during natural leaf senescence in winter wheat:Multivariate analysis as a tool for phenotypic characterization[J]. Photosynthetica2020, 58(2):301-313
[7] Faria-SilvaL, Gallon C Z, Silva D M. Photosynthetic performance is determined byscion/rootstock combination in mango seedling propagation[J]. ScientiaHorticulturae, 2020, 265: 109247.
[8] Banr A,Bruggemann W. Special issue in honour of Prof. Reto J. Strasser–Comparativeanalysis of drought stress response of maize genotypes using chlorophyllfluorescence measurements and leaf relative water content[J]. Photosynthetica,2020, 58(SPECIAL ISSUE): 638-645.
[9] CarreirasJ, Pérez-Romero J A, Mateos-Naranjo E, et al. The effect of heavy metalcontamination pre-conditioning in the heat stress tolerance of native andinvasive Mediterranean halophytes[J]. Ecological Indicators, 2020, 111: 106045.
[10] BlackhallV, Orioli G A, Colavita M G. JIP-test parameters to study apple peelphotosystem II behavior under high solar radiation stress during fruitdevelopment[J]. Photosynthetica 2020, 58(2):314-322
[11] SwoczynaT, Latocha P. Monitoring seasonal damage of photosynthetic apparatus in maturestreet trees exposed to road-side salinity caused by heavy traffic[J]. Photosynthetica58 (SI): 388-399, 2020
[12] Gast A,Romermann C, Bucher S F. Seasonal variation and trade-off between frostresistance and photosynthetic performance in woody species[J]. Photosynthetica2020, 58(2):331-340
[13] WiewióraB, Żurek G, Rybka K, et al. The origin of Epichloë endophyte-perennial ryegrasssymbionts modify plant reactions to elevated concentration of Pb2+, Cd2+ andCu2+ ions in soil[J]. BMC Plant Biology,2020.
[14] Samborska-SkutnikI A, Kalaji H M, Sieczko L, et al. Special issue in honour of Prof. Reto J.Strasser–Structural and functional response of photosynthetic apparatus ofradish plants to iron deficiency[J]. Photosynthetica 2020, 58(2):205-213
[15] Janka E,Umetani I, Sposob M, et al. Special issue in honour of Prof. Reto J.Strasser–Photosynthesis response of microalgae (Tetradesmus wisconsinensis) todifferent inorganic carbon sources probed with chlorophyll fluorescenceanalysis[J].Photosynthetica 2020, 58(2):236-244
[16] Galic V,MAZUR M, ŠIMIĆ D, et al. Special issue in honour of Prof. Reto J.Strasser–Plant biomass in salt-stressed young maize plants can be modelledwithphotosynthetic performance[J]. Photosynthetica 2020, 58(2):194-204
[17] ZhiponovaM, Paunov M, Anev S, et al. JIP-test as a tool for early diagnostics of plantgrowth and flowering upon selected light recipe[J]. Photosynthetica 58 (SI):399-408, 2020
[18] Arslan Ö,Nalcaiyi A S B, Erdal Ş Ç, et al. Analysis of drought response of sunflowerinbred lines by chlorophyll a fluorescence induction kinetics[J]. Photosynthetica2020, 58(2):348-357
[19] SwoczynaT, Łata B, Stasiak A, et al. JIP-test in assessing sensitivity to nitrogendeficiency in two cultivars of Actinidia arguta (Siebold et Zucc.) Planch. exMiq[J]. Photosynthetica, 2019, 57(2): 646-658.
[20] SamborskaI A, Kalaji H M, Sieczko L, et al. Can just one-second measurement ofchlorophyll a fluorescence be used to predict sulphur deficiency in radish(Raphanus sativus L. sativus) plants[J]. Current Plant Biology, 2019, 19:100096.
[21] Galić V,Mazur M, Šimić D, et al. Plant biomass in salt-stressed young maize plants canbe modelled with photosynthetic performance[J]. Photosynthetica, 2019, 57:9-19.
[22] Faria-SilvaL, Gallon C Z, Filgueiras P R, et al. Irrigation improves plant vitality inspecific stages of mango tree development according to photosyntheticefficiency[J]. Photosynthetica, 2019, 57(3): 820-829.
[23] PietriniF, Carnevale M, Beni C, et al. Effect of Different Copper Levels on Growth andMorpho-Physiological Parameters in Giant Reed (Arundo donax L.) inSemi-Hydroponic Mesocosm Experiment[J]. Water, 2019, 11(9): 1837.
[24] Xie Y,Sun X, Feng Q, et al. Comparative physiological and metabolomic analyses revealmechanisms of Aspergillus aculeatus-mediated abiotic stress tolerance in tallfescue[J]. Plant Physiology and Biochemistry, 2019, 142: 342-350.
[25] RusinowskiS, Szada-Borzyszkowska A, Zieleźnik-Rusinowska P, et al. How autochthonousmicroorganisms influence physiological status of Zea mays L. cultivated onheavy metal contaminated soils [J]. Environmental Science and PollutionResearch, 2019, 26(5): 4746-4763.
[26] Fusaro L,Salvatori E, Mereu S, et al. Photosynthetic traits as indicators forphenotyping urban and peri-urban forests: A case study in the metropolitan cityof Rome[J]. Ecological indicators, 2019, 103: 301-311.
[27] Pérez-RomeroJ A, Duarte B, Barcia-Piedras J M, et al. Investigating the physiologicalmechanisms underlying Salicornia ramosissima response to atmospheric CO2enrichment under coexistence of prolonged soil flooding and saline excess[J].Plant physiology and biochemistry, 2019, 135: 149-159.
[28] de SouzaLopes J, da Costa K C P, Fernandes V S, et al. Functional traits associated tophotosynthetic plasticity of young Brazil nut (Bertholletia excelsa) plants[J].Flora, 2019, 258: 151446.
[29] Bąba W,Kompała-Bąba A, Zabochnicka-Świątek M, et al. Discovering trends inphotosynthesis using modern analytical tools: More than 100 reasons to usechlorophyll fluorescence[J]. 2019.Photosynthetica 57(2):668-679
[30] Yang T Y,Cai L Y, Qi Y P, et al. BioMed Research International Increasing NutrientSolution pH Alleviated Aluminum-induced Inhibition of Growth and Impairment ofPhotosynthetic Electron Transport Chain in Citrus sinensis Seedlings[J]. BioMedResearch International,2019
[31] Çicek N,Kalaji H M, Ekmekci Y. Probing the photosynthetic efficiency of some Europeanand Anatolian Scots pine populations under UV-B radiation using polyphasicchlorophyll a fluorescence transient[J]. Photosynthetica 2020, 58(2):468-478
[32] Kalaji HM, Račková L, Paganová V, et al. Can chlorophyll-a fluorescence parameters beused as bio-indicators to distinguish between drought and salinity stress inTilia cordata Mill [J]. Environmental and Experimental Botany, 2018, 152:149-157.
[33] SamborskaI A, Kalaji H M, Sieczko L, et al. Structural and functional disorder in thephotosynthetic apparatus of radish plants under magnesium deficiency[J].Functional Plant Biology, 2018, 45(6): 668-679.
[34] Boguszewska‐Mańkowska D, Pieczyński M, Wyrzykowska A, et al. Divergent strategies displayed bypotato (Solanum tuberosum L.) cultivars to cope with soil drought[J]. Journalof agronomy and crop science, 2018, 204(1): 13-30.
[35] Kalaji HM, Bąba W, Gediga K, et al. Chlorophyll fluorescence as a tool for nutrientstatus identification in rapeseed plants[J]. Photosynthesis research, 2018, 136(3):329-343.
[36] Duarte B,Cabrita M T, Vidal T, et al. Phytoplankton community-level bio-opticalassessment in a naturally mercury contaminated Antarctic ecosystem (DeceptionIsland) [J]. Marine environmental research, 2018, 140: 412-421.
[37] Arslan Ö,Eyidoğan F, Ekmekçi Y. Freezing tolerance of chickpea: biochemical andmolecular changes at vegetative stage[J]. Biologia Plantarum, 2018, 62(1):140-148.
[38] DigradoA, Bachy A, Mozaffar A, et al. Long‐term measurements of chlorophyll a fluorescence using the JIP‐test show that combined abiotic stressesinfluence the photosynthetic performance of the perennial ryegrass (Loliumperenne) in a managed temperate grassland[J]. Physiologia plantarum, 2017,161(3): 355-371.
[39] MarcińskaI, Czyczyło-Mysza I, Skrzypek E, et al. Application of photochemical parametersand several indices based on phenotypical traits to assess intraspecificvariation of oat (Avena sativa L.) tolerance to drought[J]. Acta PhysiologiaePlantarum, 2017, 39(7): 153.
[40] DigradoA, Gourlez de la Motte L, Bachy A, et al. Long-term field study of theinfluence of the photosynthetic performance of temperate grassland species onecosystem CO2 exchange fluxes at the ecosystem-scale[J]. Gembloux Agro-biotech,2017.
[41] Yang X Q,Zhang Q S, Zhang D, et al. Light intensity dependent photosynthetic electrontransport in eelgrass (Zostera marina L.) [J]. Plant Physiology andBiochemistry, 2017, 113: 168-176.
[42] SemerciA, Semerci H, Çalişkan B, et al. Morphological and physiological responses todrought stress of European provenances of Scots pine[J]. European Journal ofForest Research, 2017, 136(1): 91-104.
[43] MajewskaM L, Rola K, Zubek S. The growth and phosphorus acquisition of invasive plantsRudbeckia laciniata and Solidago gigantea are enhanced by arbuscularmycorrhizal fungi[J]. Mycorrhiza, 2017, 27(2): 83-94.
[44] Kalaji HM, Schansker G, Brestic M, et al. Frequently asked questions about chlorophyllfluorescence, the sequel[J]. Photosynthesis Research, 2017, 132(1): 13-66.
[45] DąbrowskiP, Baczewska A H, Pawluśkiewicz B, et al. Prompt chlorophyll a fluorescence asa rapid tool for diagnostic changes in PSII structure inhibited by salt stressin Perennial ryegrass[J]. Journal of Photochemistry and Photobiology B:Biology, 2016, 157: 22-31.
[46] Kalaji HM, Jajoo A, Oukarroum A, et al. Chlorophyll a fluorescence as a tool to monitorphysiological status of plants under abiotic stress conditions[J]. Actaphysiologiae plantarum, 2016, 38(4): 102.
[47] PollastriniM, Holland V, Brüggemann W, et al. Taxonomic and ecological relevance of thechlorophyll a fluorescence signature of tree species in mixed Europeanforests[J]. New Phytologist, 2016, 212(1): 51-65.
[48] Canton GC, Bertolazi A A, Cogo A J D, et al. Biochemical and ecophysiological responsesto manganese stress by ectomycorrhizal fungus Pisolithus tinctorius and inassociation with Eucalyptus grandis[J]. Mycorrhiza, 2016, 26(5): 475-487.
[49] JurczykB, Rapacz M, Pociecha E, et al. Changes in carbohydrates triggered by lowtemperature waterlogging modify photosynthetic acclimation to cold in Festucapratensis[J]. Environmental and experimental botany, 2016, 122: 60-67.
[50] Bąba W,Kalaji H M, Kompała-Bąba A, et al. Acclimatization of photosynthetic apparatusof tor grass (Brachypodium pinnatum) during expansion[J]. PloS one, 2016,11(6).
[51] Ogar A,Sobczyk Ł, Turnau K. Effect of combined microbes on plant tolerance to Zn–Pbcontaminations[J]. Environmental Science and Pollution Research, 2015, 22(23):19142-19156.
[52] Østrem L,Rapacz M, Larsen A, et al. Influences of growth cessation and photoacclimationon winter survival of non-native Lolium–Festuca grasses in high-latituderegions[J]. Environmental and Experimental Botany, 2015, 111: 21-31.
[53] Fusaro L,Salvatori E, Mereu S, et al. Urban and peri-urban forests in the metropolitanarea of Rome: Ecophysiological response of Quercus ilex L. in two greeninfrastructures in an ecosystem services perspective[J]. Urban Forestry &Urban Greening, 2015, 14(4): 1147-1156.
[54] Tyrka M,Rapacz M, Fiust A, et al. Quantitative trait loci mapping of freezing toleranceand photosynthetic acclimation to cold in winter two‐and six‐rowed barley[J]. Plant Breeding, 2015, 134(3): 271-282.
[55] Kalaji HM, Oukarroum A, Alexandrov V, et al. Identification of nutrient deficiency inmaize and tomato plants by in vivo chlorophyll a fluorescence measurements[J].Plant physiology and biochemistry, 2014, 81: 16-25.
[56] GiovenzanaV, Beghi R, Buratti S, et al. Monitoring of fresh-cut Valerianella locustaLaterr. shelf life by electronic nose and VIS–NIR spectroscopy[J]. Talanta,2014, 120: 368-375.
[57] Żurek G,Rybka K, Pogrzeba M, et al. Chlorophyll a fluorescence in evaluation of theeffect of heavy metal soil contamination on perennial grasses[J]. Plos one,2014, 9(3).
[58] SalvatoriE, Fusaro L, Gottardini E, et al. Plant stress analysis: Application of prompt,delayed chlorophyll fluorescence and 820 nm modulated reflectance. Insightsfrom independent experiments[J]. Plant physiology and biochemistry, 2014, 85:105-113.
[59] Zurek G,Rybka K, Pogrzeba M, et al. Chlorophyll a Fluorescence in Evaluation of theEffect of Heavy Metal Soil Contamination[J]. Plos one,2014.
[60] BlumenthalJ, Megherbi D B, Lussier R. Unsupervised machine learning via Hidden MarkovModels for accurate clustering of plant stress levels based on imagedchlorophyll fluorescence profiles & their rate of change in time[C]//2014IEEE International Conference on Computational Intelligence and VirtualEnvironments for Measurement Systems and Applications (CIVEMSA). IEEE, 2014:76-81.
[61] VanHeerden P D R. Differential acclimation capacity to frost in sugarcanevarieties grown under field conditions[J]. Plant Growth Regulation, 2014,72(2): 181-187.
[62] PotgieterC, De Beer M, Claassens S. The effect of canola (Brassica napus) as abiofumigant on soil microbial communities and plant vitality: a pot study[J].South African Journal of Plant and Soil, 2013, 30(4): 191-201.
[63] BresticM, Zivcak M. PSII fluorescence techniques for measurement of drought and hightemperature stress signal in crop plants: protocols andapplications[M]//Molecular stress physiology of plants. Springer, India, 2013:87-131.
[64] GoltsevV, Zaharieva I, Chernev P, et al. Drought-induced modifications ofphotosynthetic electron transport in intact leaves: analysis and use of neuralnetworks as a tool for a rapid non-invasive estimation[J]. Biochimica etBiophysica Acta (BBA)-Bioenergetics, 2012, 1817(8): 1490-1498.
[65] Jones MO, Piron-Prunier F, Marcel F, et al. Characterisation of alleles of tomatolight signalling genes generated by TILLING[J]. Phytochemistry, 2012, 79:78-86.
[66] GoltsevV, Zaharieva I, Chernev P, et al. Drought-induced modifications ofphotosynthetic electron transport in intact leaves: analysis and use of neuralnetworks as a tool for a rapid non-invasive estimation[J]. Biochimica etBiophysica Acta (BBA)-Bioenergetics, 2012, 1817(8): 1490-1498.
[67] MaboetaM, van Rensburg L. Vermicomposting of Industrial Organic Wastes and itsApplication in Mine Rehabilitation Strategies–An Overview from a South AfricanPerspective[J].2012
[68] Wagg S,Mills G, Hayes F, et al. OZONE AND DROUGHT STRESS INTERACTIONS IN BEAN ANDGRASSLAND SPECIES[J]. Programme &, 2011: 33.
[69] PollastriniM, Desotgiu R, Cascio C, et al. Growth and physiological responses to ozone andmild drought stress of tree species with different ecological requirements[J].Trees, 2010, 24(4): 695-704.
[70] Torres-RomeroD, King-Diaz B, Strasser R J, et al. Friedelane triterpenes from Celastrusvulcanicola as photosynthetic inhibitors[J]. Journal of agricultural and foodchemistry, 2010, 58(20): 10847-10854.
[71] Kirova M,Ceppi G, Chernev P, et al. Using artificial neural networks for plant taxonomicdetermination based on chlorophyll fluorescence induction curves[J].Biotechnology & Biotechnological Equipment, 2009, 23(sup1): 941-945.
[72] Pérez-MéndezA, Maldonado-Rodríguez R, Torres-Rivas E, et al. Pisum sativum classificationbased on a methodological approach for pattern recognition using discriminantanalysis and neural networks[J]. stress, 2005, 8(17): 18.
[73] Soja G,Soja A. Recognizing the sources of stress in wheat and bean by usingchlorophyll fluorescence induction parameters as inputs for neural networkmodels[J]. PHYTON-HORN-, 2005, 45(3): 157.