New Brunswick S41i CO2 恒温摇床进行间充质干细胞扩增(二)
Stem cell differentiation assays
AdMSCs were harvested from both shake and spinner flasks into 50 ml
tubes. Once the microcarrier beads settled to the bottom of the tube,
the supernatants were removed and cells were washed with DPBS.
Afterwards, the microcarrier beads were treated with 5 ml of prewarmed
trypsin-EDTA solution at 37 °C for 10 min. During incubation, the tubes
were occasionally vortexed for 2 sec and then neutralized by adding an
equal volume of trypsin neutralizing solution. Microcarrier beads were a
llowed to settle to the bottom of the tube and the supernatants were
collected as soon as possible. Microcarrier beads were washed 2-3 times
with DPBS and as much supernatant as possible was collected into a 50 ml
tube. Following washing, AdMSCs were collected to bottom of the tube by
centrifugation at 120 xg for 5 min and resuspended in 5 ml of
mesenchymal stem cell medium. Cells were seed at a density of 18,000
cells/cm2 into a 24-well plate. Adipocytes and osteocyte differentiations
were performed on those cells using differentiation assay kits from
ATCC. Adipocyte and osteocyte differentiated cells were identifed by
cell-type specifc staining with either Oil red O or Alizarin red S kits
(ScienCell®) according to manufacturer instructions and visualized using an OLYMPUS® CK40 microscope.
Results and Discussion
To compare between shake flask and spinner flask cultures, AdMSCs were
seeded at a density of 3x103 cells/cm2 in both systems. Cell culture
comparisons were conducted for 12 days and samples were collected for
cell growth, biochemistry and metabolite analysis daily. Cell growth
comparisons revealed that AdMSCs cultured under shake flask conditions
achieved between 1.6 and 1.8-fold higher cell count over spinner flask
cultures during early log phase (day 4) and stationary phase (day 9) of
growth (Figure 1A). Biochemistry and metabolite analysis revealed that
glucose concentrations decreased from 1.09 g/l to 0.548 g/l (for shake
flask culture) and 0.798 g/l (for spinner culture), whereas lactate
concentrations increased from 0.042 g/l to 0.396 g/l (for shake flask
culture) and 0.259g/l (for spinner culture) after 12 days of culture
(Figure 1B &C). The higher glucose consumption and lactate
production rate seen in the shake flask culture supports the fnding that
the stem cells grew at a faster rate under the shake flask conditions.
Furthermore, during early growth phase (day 4); the amount of ammonium
accumulated in spinner flask culture (2.4 mM) was 1.8-fold higher than
shake flask culture (1.3 mM) (Figure 1D). It has been shown that even low
level of ammonium (1.9 mM) inhibits MSC growth5. The spinner culture
has shown ammonium level exceeding 2 mM early and throughout the culture
process, which indicates the slower growth by the spinner method could
be a result of ammonium toxicity-induced growth inhibition. The fact
that spinner culture had elevated ammonium levels early in the culture
not seen in the shake flask also indicates possible stem cell damage due
to shear force by the spinner rod. The spinner rod was observed to
display a “stop & go” motion at low speeds; precise speed control is
not possible, especially at low rotation speeds.
To determine whether or not AdMSCs retained their stem cell properties
during their growth under shake flask conditions, immunostaining of stem
cell surface markers and differentiation assays were performed.
Microcarrier beads that contained AdMSCs were immunostained with stem
cell surface marker antibodies such as: FITC conjugated antihuman CD44
and APC-conjugated antihuman CD90 and revealed that AdMSCs retained stem
cell surface markers during growth under shake flask culture condition
(Figure 2A&B). For the adipocyte and osteocyte differentiation
assays, AdMSCs were collected from the microcarrier beads and seeded
into 24 well plates that contained either adipocyte or osteocyte
differentiation medium. After 17 days of culture, the plates were stained
with Oil Red O or Alizarin Red S staining solutions, respectively.
Microscopic observation indicated that most of the AdMSCs from shake
flask culture differentiated into either adipocytes or osteocytes
successfully (Figure 3A&B).
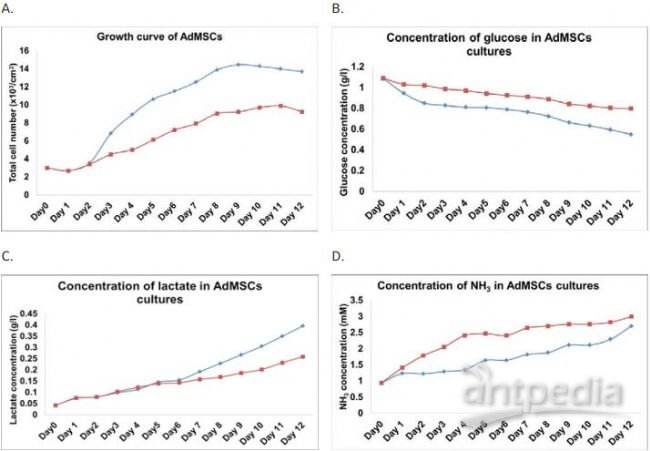
Figure 1. Comparison of AdMSCs between
shake fl ask and spinner fl ask cultures: A) growth; B) glucose
utilization; C) lactate production and D) ammonium production.() shake fl
ask and () spinner fl ask.

Figure 2. Stem cell marker identif cation
assay for AdMSCs expanded on microcarriers in shake fl ask. A) AdMSCs on
microcarrier beads are positive for CD90 stem cell marker, as indicated
in red by fl uorescence imaging. B) AdMSCs on microcarrier beads are
positive for CD 44 stem cell marker, as indicated in green by
Fluorescence Imaging. Blue color indicates stem cell nuclear staining by
DAPI.
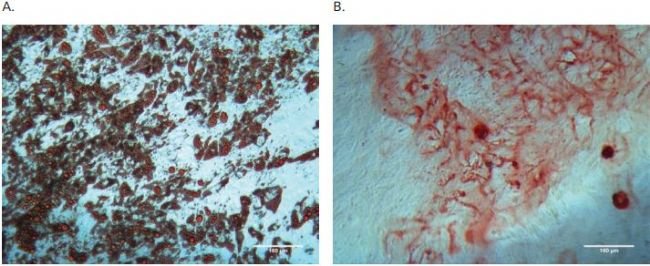
Figure 3. Diff erentiation assays for AdMSCs expanded on microcarriers in shake fl ask. A) Adipogenic diff erentiation formed lipid droplets as indicated by Oil red O positive staining. B) Osteogenic diff erentiation caused calcium mineralization of extracellular matrix as indicated by Alizarin Red S positive staining.
Conclusions
Stem cell expansion using shake flask conditions appears to be a viable and simple alternative to the spinner flask system. This novel method relies on a new type of CO2 incubator with built-in shaking capability, such as the New Brunswick S41i CO 2 Incubator Shaker. The New Brunswick S41i reduces shearing, eliminates potential cell damage by the spinner rod, decrease the risk of contamination associated with inserting a magnetic stirrer base into the CO 2 incubator and reduces experimental complexity. This method also greatly increases the cell culture capacity whereby a large number of shake flasks can be placed in the New Brunswick S41i simultaneously. In the case of spinner flask culture, a typical incubator without active cooling can only handle the heat emitted from a very limited number of magnetic stirrer bases before causing temperature setpoint overshoot, a signifcant limitation to the scale-up potential of the spinner method. This reinforces the superiority of New Brunswick S41i CO 2 Incubator Shaker as an alternative to incubator/spinner based stem cell culture. This method eliminates a scale-up bottleneck while providing the highest quality stem cell culture for inoculation of large scale industrial bioreactors for the production of clinical material.
References
1. Zhang et al. “Mechanisms Underlying the Osteo- and
Adipo-Differentiation of Human Mesenchymal Stem Cells”. The Scientifc
World Journal (2012).Volume 2012; Article ID793823;
2. Nastran el al. “Osteogenic Differentiation and Osteochondral Tissue
Engineering Using Human Adipose-Derived Stem Cells.” Biotechnology
progress (2013) Volume 29.No.1: 176- 185;
3. Schop et al. “Expansion of human mesenchymal stromal cells on
microcarriers: growth and metabolism” J. Tissue Eng Regen Med (2010);
Volume
4. No. 4; 131-140. 4. Kohlstrom et al. “Hybridoma and CHO Cell Culture
using New Brunswick S41i – An Environmental Friendly, Low Emission
Incubator Shaker”. Eppendorf Application Note AA257, Nov. 2012.
5. Schop D. “Growth and Metabolism of Mesenchymal Stem Cells Cultivated
on Microcarriers”, Ph.D. Thesis 2010, University of Twente, The
Netherlands.