罗氏收购Ariosa Diagnostics 立足无创产前筛查市场
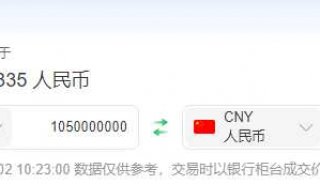
北京时间2014年12月2日,罗氏宣布将要收购无创产前筛查技术的提供商Ariosa公司。收购价格暂未公布,预计收购将于本月完成。
Ariosa提供的Harmony产前测试,是一种血液测试21、18 和13三染色体性风险,以及从胎儿循环中测性染色体异常和从怀孕10周起测试孕妇无细胞DNA。不同于其他NIPT测试,这种分析总DNA的Harmony测试采用有针对性的着重感兴趣的染色体。
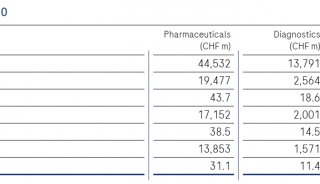
罗氏首席运营官(COO)Roland Diggelmann提到,并购Ariosa是罗氏致力于先进分子诊断技术的又一实例,循环游离DNA技术能够在很多重要的诊断中提供诊断信息,例如怀孕、癌症和器官移植。
Ariosa于2012年开始Harmony产前检测技术实验,至2014年5月,该公司已经在全球超过90个国家执行了225,000次检测。
虽然目前实验是基于竞争对手Illumina提供的基因测序技术,但是Ariosa公司说他们近期开发出的一种基于微阵列的方法也可以用于实验,并且在早前已经跟Affymetrix公司签订了一份长期的合作协议。
在2012年初,Ariosa公司在C轮融资中获得了5270万美元融资,投资方包括Venrock, Domain Associates, Meritech Capital Partners以及一个没有披露名称的共同基金。
Ariosa目前与Sequenom、Illumina公司存在大量的ZL侵权诉讼。
Roche to Acquire Ariosa Diagnostics to Gain Foothold in NIPT, Cell-free DNA Testing Markets
NEW YORK (GenomeWeb) — Roche will acquire non-invasive prenatal testing provider Ariosa Diagnostics of San Jose, Calif.
The purchase price was not immediately available, and the acquisition is expected to close this month.
Ariosa offers the Harmony Prenatal Test, a blood test that gauges the risk of trisomies 21, 18, and 13 as well as sex chromosome abnormalities from circulating fetal and maternal cell-free DNA starting at 10 weeks of pregnancy. Unlike some other NIPT that analyze total DNA, the Harmony test uses a targeted approach that focuses on DNA from the chromosomes of interest.
"The acquisition of Ariosa is another example of Roche's commitment to advanced molecular diagnostics," said Roland Diggelmann, COO of Roche Diagnostics, in a statement. He added that circulating cell-free DNA promises to provide diagnostic information "in many important segments," including pregnancy, cancer, and transplantation.
Ariosa launched the Harmony test, which is performed in its CLIA laboratory, in 2012, marketing it through LabCorp's Integrated Genetics business in the US and Canada. As of May 2014, the company had conducted 225,000 Harmony tests worldwide and was offering it in more than 90 countries.
The test currently runs on sequencing technology from competitor Illumina, but Ariosa said recently that it has developed a microarray-based method to carry out the test and struck a multi-year supply agreement with Affymetrix for arrays and instruments this fall.
The company was founded as Tandem Diagnostics before it changed its name to Aria Diagnostics, then Ariosa Diagnostics.
In early 2012, the firm raised $52.7 million in a Series C financing round that included investors Venrock, Domain Associates, Meritech Capital Partners, and an undisclosed mutual fund group.
Ariosa is embroiled in a number of patent infringement lawsuits, including with Sequenom, Illumina, and Illumina's Verinata Health business.