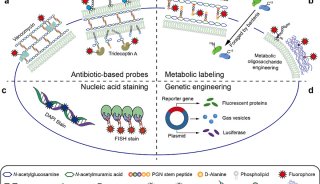
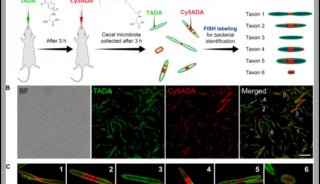
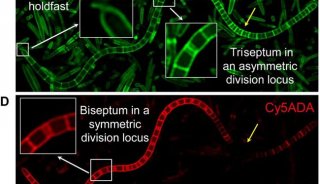
FDA批准首个可鉴别特异性致病菌检测法上市
美国食品与药物管理局(FDA)于2012年6月27日批准首个可用于鉴定引起血流感染的12种不同细菌型的核酸检测上市。该项检测被命名为Verigene GP血培养核酸检测(BC-GP),能够在首次观察到细菌增殖征象后数小时内,同时检测细菌类别和3个相关耐药基因。而传统细菌鉴定方法则需要2至4天才能获得同样的结果。
该检测方法可鉴别不同的葡萄球菌属(包括耐甲氧西林金葡菌,MRSA),链球菌、肠球菌(包括耐万古霉素肠球菌,VRE)以及李斯特菌。
FDA医疗器械和辐射健康中心主任Alberto Gutierrez指出:“血流感染通常需要进行抗生素治疗,尽快为不同的患者选择特定的抗生素是治疗的根本,这项检测手段能够帮助医生快速为患者选择正确的抗生素。
血流感染是美国医院最常见和最严重的疾病之一。细菌进入血流能够导致严重的疾病,包括心脏、肾脏和其它重要器官的感染。如果不进行治疗这些重要脏器的感染会导致包括死亡在内的严重后果。
FDA做出这项决定是基于一项纳入1,642例患者血样的研究,这些样本血培养均发现革兰阳性细菌。该研究对比了BC-GP和传统的实验室血培养方法,结果表明BC-GP与后者的检测结果吻合度为93~100%。
FDA allows marketing of first test to identify certain bacteria associated with bloodstream infections
Test makes it possible to identify potentially serious illness causing bacteria hours after a positive blood culture
The U.S. Food and Drug Administration today allowed marketing of the first nucleic acid test that can identify 12 different bacterial types known to cause bloodstream infections. The test allows for simultaneous identification of the bacteria and three associated resistance genes in just a few hours after the first sign of bacterial growth. Traditional methods may require two to four days to produce bacterial identification and resistance results.
The Verigene GP Blood Culture Nucleic Acid Test (BC-GP) can identify different types of Staphylococcus, (including methicillin-resistant Staphylococcus aureus or MRSA), Streptococcus, Enterococcus (including vancomycin-resistant Enterococci or VRE), and Listeria.
“Bloodstream infections are always treated with antimicrobial drugs, and it is essential to identify which antimicrobial drug is appropriate for a specific patient as quickly as possible,” said Alberto Gutierrez, director of the Office of In Vitro Diagnostic Device Evaluation and Safety at the FDA’s Center for Devices and Radiological Health. “This new test is an important tool that will help physicians treat patients quickly with the correct antibiotics.”
Bloodstream infections are one of the most common and serious illnesses found in U.S. hospitals. Bacteria entering the bloodstream can cause severe illness, including infections of the heart, kidney, and other vital organs. Without treatment, infection of vital organs can result in serious consequences, including death.
The FDA based its decision on a study of 1,642 patient blood samples obtained from incubated blood culture bottles that contained gram positive bacteria. The study included a comparison of BC-GP and traditional blood culture laboratory methods. The BC-GP results were consistent with traditional blood culture methods in 93 percent to 100 percent of the comparisons.
The Verigene is manufactured by Nanosphere Inc. of Northpook, Ill.
http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm309
-
项目成果
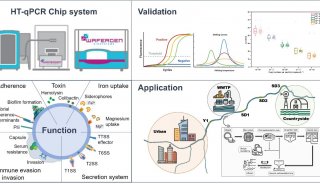
-
科技前沿