A study of Fetal Alcohol Syndrome
Introduction
Fetal Alcohol Syndrome (FAS) is caused by exposure of the developing embryo to alcohol, one of several teratogenic agents which adversely affect the developing embryo. FAS is one of the most common birth defects in the Western world, specifically characterized by growth and mental retardation, craniofacial malformations, and heart and neural defects (Gilbert, 1997; Cartwright and Smith, 1995; Smith, 1997; Sulik et al., 1988). Ethanol exposure is estimated to severely affect 1 in 1000 human births and to have lesser or associated effects in 3-4 in 1000 human births (Sulik et al., 1988). It has been demonstrated that the effects of FAS in mouse and chick models are comparable to those in humans, and these organisms serve as valuable mechanistic models to examine the effects of teratogents such as alcohol on tissue development (Cartwright and Smith, 1995 and Sulik et al., 1988). Studies using non-human models, such as the chick, can be used to help promote the understanding and future treatment of this preventable condition.
Many of the structures affected by FAS stem from neural crest cells (NCC), a group of cells derived from the dorsal neuroectoderm during embryogenesis (Gilbert, 1997). Cartwright and Smith (1995) observed increased aberrant cell death of NCC after ethanol exposure in chicks. Sulik et al. (1988) also observed increased cell death after exposure to ethanol in mammalian models. The cranial neural crest, which leads to the formation of the entire facial skeleton, is an especially sensitive target of ethanol-induced apoptosis (Ahlgren et al., 2002). These research teams suggest that this increased NCC death may be responsible for FAS phenotypes.
More recent research has explored the molecular causes of FAS. Expression of msx2, a homeobox gene, is an integral factor of the development of craniofacial structure and the developing brain. Expression of msx2 was found to decrease in mice exposed to alcohol (Rifas et al., 1997). The misexpression of this gene corresponds to the affected FAS phenotypic areas seen in previous research (Rifas et al., 1997). Blader and Strähle (1998) found phenotypic disturbances to be the result of misexpression of several ventral brain markers, includingshh, axl, and nk2.2.
Cell death is observed in the premigratory and migratory neural crest cells, similar to an observation made after blocking Sonic hedgehog (Shh) signaling (Ahlgren et al., 2002). Ahlgren and colleagues demonstrated that ethanol exposure results in a loss of Shh and transcripts in the Shh signaling pathway, and that ethanol-induced cranial NCC apoptosis and associated growth defects can be salvaged by application of Shh.
Teratogens can also have different effects on the developing embryo depending on the magnitude and time of dose. Trunk NCC are precursors for the peripheral nervous system, ganglia, and glial cells. They are determined relatively late in migration, and thus are multipotent and can compensate for some cell death. Alcohol does not seem to affect these cells and their future structures to the same degree as the cranial NCC (Smith, 1997). It has been found in chick embryos that alcohol causes apoptosis of craniofacial cells only if treatment was administered before the emigration of NCC from the neurectoderm, at approximately 18 to 36 hours of development (Smith, 1997). Since the fate of these cells are determined at emigration, compensation for lost cells cannot occur in the same manner as the trunk NCC and deformities are observed (Bronner-Fraser and Fraser, 1991; Smith, 1997).
Cell death induced by alcohol may not occur until 46-48 hours of development (Smith, 1997). To observe areas of apoptosis, Fallon and Saunders (1968) first reported the method of using the vital dye Neutral Red, and this method has been published in recent works to reveal the extent of cell death in tissues (Garcia-Martinez et al., 1993; Kim and Mirkes, 2003).
Based on this knowledge, our experiment investigates the phenotypic responses of FAS at different treatment doses and times in the chick model. Apoptosis in response to ethanol treatment is visualized using Neutral Red staining techniques.
Purpose
This lab has three purposes. Our primary objective is to observe the later stage effects of a single exposure of ethanol to chicks early in development. We will look for phenotypic anomalies and compare our results to previous research. Second, we explore whether a dose dependence exists in the morphological effects of FAS. Previous work has not presented a conclusive model for dose dependence of a single injection of ethanol. We expect to see greater malformations with increased concentrations of ethanol. Our third goal is to investigate whether effects are dependent on the time of treatment. We expect to see greater extent of malformations in embryos that were treated with ethanol during a "critical window" of sensitivity at between 18-36 hours into development, when the cranial NCC are emigrating.
Materials and Methods
Following the methods described in Cartwright and Smith (1995), we staged several 27 hour chick embryos according to the system by Hamburger and Hamilton (1951) and prepared them for injection by creating a small hole in the blunt end of the egg. A 250 µl injection of 0% (control), 5%, 10%, or 15% ethanol (in Howard Ringer's solution) was introduced directly into the yolk using a 1 ml syringe. Each concentration of ethanol was injected into a set of ten eggs. Eggs which were not viable or died before collection were discarded and eliminated as data.
The eggs were then incubated at 37ºC for 14 days. At day 14 post-injection, the development of the embryos was terminated and the effects of the ethanol were noted. Eggs were opened carefully and chicks were qualitatively compared to one another. Additionally, quantitative data was gathered. The total body mass, head diameter, and beak length were measured for each set of embryos. Head diameter was measured with calipers which were placed immediately behind the eyes. Calipers were also used to measure beak length from the tip of the beak to the end of the beak.
The data was analyzed using Data Desk software. First, an ANOVA test was performed. A Bonferroni post hoc analysis of the data was carried out. The results of these tests were used to determine whether any significant trends in our data existed.
Temporal -dependence
Swarthmore College
We obtained 24 27-hour eggs and 12 51-hour eggs. One egg from each age was opened and staged according to Hamburger and Hamilton (1951). Of the remaining eggs, each was sterilized on the blunt end with 70% ethanol and a small hole was made using beak forceps. Through the hole, 8 27-hour eggs and 5 51-hour eggs, designated the controls, were injected with 250 µl Howard Ringer's solution directly into the yolk using a 1 mL syringe. The remaining eggs were injected in the same manner with 15% ethanol in Howard Ringer's solution, determined by taking the dose that produced the most significant effects in the dose-dependent experiment. Within our experiment, only a few of the eggs were viable at harvesting and it was hypothesized that the needle was injected directly into the blastodisc, terminating development. Allowing the blastodisc to float to the top and rotating the blunt end of the egg to the side to allow for injection away from the blastodisc and into the yolk may reduce this problem. The holes were then covered with Scotch tape to minimize infection and eggs were incubated at 37ºC.
Forty-eight hours post-injection, one experimental and one control egg from each age group was harvested and treated with Neutral Red vital stain to detect apoptosis. Neutral Red stock solution was diluted 1:100 in Howard Ringer's sollution and embryos were incubated in the dye for 20 minutes at 37ºC, after which they were rinsed in Howard Ringer's solution and photographed using the Olympus DP 12.
At ten days post-injection, the development of the eggs treated at 51-hours was examined by carefully opening the eggs and isolating the embryo from the rest of the egg. Mass, head diameter, and beak length were recorded and the embryos were again photographed. In consistency with the above study, head diameters were measured with calipers placed immediately behind the eyes, and beak lengths were measured from the tip to end of the beak.
Eggs treated at 27-hours were harvested 11 days post-injection to be consistent that all embryos were the same developmental age to control differences in size and mass measurements. Data were collected as above.
Results
Upon initial qualitative analysis, definite morphological differences were noted in the four groups. Data were collected and statistical analysis was used to clarify our observations. Trends of decreasing mass, head diameter, and beak length with increasing ethanol dosage were observed as seen by a negative slope in regression analyses . We proceeded to determine whether these differences were significant.
No significant differences existed between any of the chicks' masses (ANOVA: F-ratio = 1.3544, p=0.2923) . A significant difference was measured in chick head diameter across the different dosage groups (ANOVA: F-ratio = 22.010, p<=0.0001) . A Bonferroni post hoc analysis indicated significant differences between 5% to control (p=0.001244), 10% to control (p=0.000020), and 15% to control (0.000067) . No significant differences were seen between any beak lengths (ANOVA: F-ratio = 3.0072, p=0.0612)
Although significant difference were not always observed, a great deal of variation within each measurement for each group was seen. illustrates the most notable superficial difference in size between a control and a 10% ethanol treated embryo. illustrates the difference in head size between a control and a 10% ethanol treated embryo.shows the difference in beak size between a control and a 10% ethanol treated embryo.
Temporal-dependence
The eggs treated earlier, at 27-hours, were smaller in mass, head diameter, and had shorter beak length than those exposed to ethanol at 51-hours or the control eggs, consistent with Lawrence and Yoder's study. There were visible malformations particularly in the craniofacial region, including missing beaks, misshaped heads, and soft skulls .
From the set of eggs treated at 51-hours, two ethanol-treated embryos and three control embryos were harvested .The eggs treated at 27-hours yielded seven experimental and one control embryo . All treated embryos were smaller in mass and head diameter and had shorter beaks compared to their respective control embryos. One embryo exposed to ethanol at 27 hours was missing its beak .
Vital dye staining with Neutral Red revealed more specific areas of apoptosis in both ethanol-treated embryos, most prominently in the midbrain area of the embryo treated at 27 hours (Figure 8). The embryo treated at 51 hours with ethanol did not exhibit staining in the head region, but rather more caudally (Figure 9). Red dye was not present in the control embryos (Figure 10).
Discussion
The effects of ethanol on body mass
The regression graphs illustrate a decrease in mass as ethanol dose increases . However, this notable decrease is not significant . The lack of a significant relationship between body mass to ethanol dose could possibly be explained by the fact that all body cells are not derived from NCC, which have been observed to be affected by ethanol (Cartwright and Smith, 1995, Sulik et al., 1988, and Blader and Strähle, 1998). A proposed, yet unsubstantiated, argument for the observed decline in mass is that perhaps all body cells are affected by ethanol treatment to varying degrees. Future research could pursue this study. An additional reason why a significant difference was not noted may be because a single dose was insufficient to obtain a critical level of ethanol to significantly affect the embryo. Continued research on increasing the concentration of ethanol or the number of injections over time could reveal that body mass is affected by ethanol treatments. We did not investigate these possibilities in this experiment. We propose continued research should use a larger sample size to determine if the observed amount of variation is acceptable or if the variation using the same protocol will, in fact, decrease.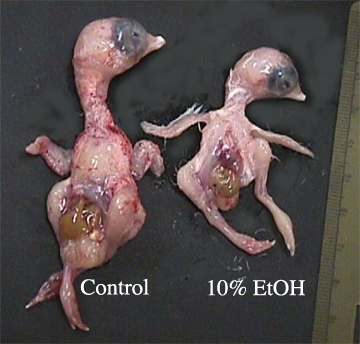
The effects of ethanol on head diameter
Since a significant difference occurred in head diameter between the different ethanol exposed groups to the control, we conclude that NCC were indeed affected by the single ethanol treatment .This supports previous research in this area (Blader and Strähle, 1998, Cartwright and Smith, 1995, Rifas et al., 1997, and Sulik et al., 1988). We conclude that a critical level of ethanol was reached because we noted significant differences among the test groups to the control. Similar to our results for body mass and beak length, a great deal of, but less, variation was observed .Continued research should use a larger sample size to determine if the observed amount of variation is acceptable or if the variation using the same protocol will decrease.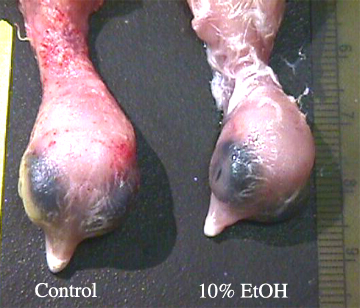
The effects of ethanol on beak length
Since beak tissue is derived from NCC (Gilbert, 1997), we expected a decrease in beak length. The regression of beak length reveals a trend toward decreasing length with increasing ethanol concentration , but these values are not significant . Perhaps the injected amount of ethanol was not significant enough to elicit the anticipated response in the developing chick embryos. Further research could explore the possibility of attaining the potential critical concentration.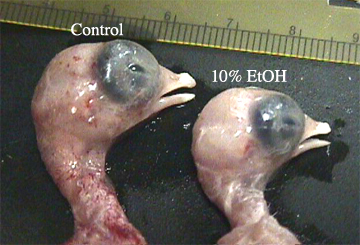
The effects of ethanol at different treatment times Only 2 of the 6 51-hour and 7 of the 15 27-hour treated eggs survived, suggesting that ethanol at 15% can cause sufficient disruptions in the Shh signaling pathway or elsewhere in the embryo such that development is terminated. Other mechanisms such as alcohol competition with retinol to bind with Class IV alcohol dehydrogenase (ADH), which leads to retinoic acid deficiency and and craniofacial defects, may also be a factor in the termination of embryos (Chen and Sulik, 1996; Deltour et al., 1996). However, only 3 of the 5 51-hour and 1 of 8 27-hour control eggs also survived, suggesting that ethanol may not have been the cause of death. The malformations were consistent with the previous findings in this survey, and we conclude that effects on beak length and head diameter are in fact due to ethanol. When injecting the eggs, there was no consideration as to where the blastodisc may be located, leading to the hypothesis that perhaps needles were inserted directly into the blastodisc, disrupting and terminating development. Within the set of harvested embryos there were notable differences in mass between the control and treated embryos in mass, head diameter, and beak length, consistent with Lawrence and Yoder's data .The presence of embryos with severe malformations including missing beaks suggests that ethanol does have a teratogenic effect, especially in the craniofacial region . Neutral Red vital dye staining revealed that NCC death occured to varying degrees when ethanol was injected at different times during development. Since chick development occurs in an anterior to posterior fashion, the most posterior cells are less mature than those in the anterior region. Early exposure to alcohol results in cell death in the head region, whereas later exposure would affect more caudal regions (Smith, 1997). Thus, the older embryo showed little to no apoptosis in the craniofacial region but some apoptosis in the more posterior regions in the spineThe embryo injected with 15% ethanol at 27 hours exhibited much staining in the craniofacial region, most prominently in the midbrain and down the spine . The control embryo displayed no staining Trunk NCC are precursors for the peripheral nervous system, ganglia, and glial cells. They are determined relatively late in migration and thus are multipotent and can compensate for some cell death. Alcohol did not seem to affect these cells and their future structures to the same degree as the cranial structures. In contrast, the cranial NCC are determined at emigration and there seems to be a critical window of 18-36 hours of incubation where these cells are most sensitive to alcohol (Smith, 1997). Our embryos treated at 27 hours fit into this critical window and we see much neural crest apoptosis Distinctive facial features characterize FAS in humans and individuals exhibit neurodevelopmental deficits (Smith, 1997). Understanding mechanisms involved in FAS may help to control or limit alcohol-induced damage to the fetus, as well as propose methods to prevent or repair damage done to the prain and central nervous system later in life, an issue of great public health importance. | |||||
Embryonic viability. | |||||
Eggs which were discarded were not counted due to a lack of viability or a lethal fungal infection (data not shown). Due to the fact that we could not control for these situations, ten data points are not present for each treatment group. Furthermore, future studies should investigate whether higher doses of ethanol cause embryonic death in which the effects are not acute. | |||||



















