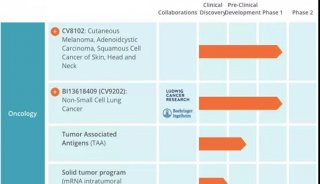
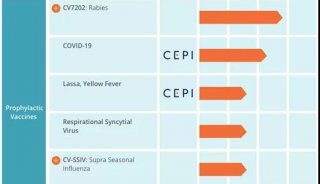
Esperion治疗低密度胆固醇药物即将进入临床三期研究
总部设在密歇根州安娜堡的生物医药公司Esperion最近迎来了2015年的第一个好消息。公司透露FDA已经批准了公司开发的胆固醇药物ETC-1002进入临床三期研究。公司表示FDA已经取消了对于ETC-1002临床研究不得超过6个月的限制。FDA此前对这种药物的毒性表示了忧虑。不过,去年Esperion公司向FDA提交了大量的临床前研究数据,使得FDA改变了这种看法。公司计划在今年招募大约4000名患者进行临床研究以检验其降低LDL胆固醇的效果。
近年来市场上不乏一些效果极佳的降LDL胆固醇药物,一些制药巨头如安进公司、赛诺菲公司都在这一领域有自己的产品。不过,这些药物大都属于昂贵的抗体药物。而ETC-1002则是一种小分子药物。公司CEO认为ETC-1002将为许多患者提供除他汀类药物以外的又一种选择,而昂贵的抗体药物如一些PCSK9抑制剂类抗体药物也将降成为历史。
作为一家小型生物医药公司,公司在去年进行了总额1亿美元的IPO,但是公司表示目前还在寻找更具经验的合作伙伴。(生物谷Bioon.com)
详细英文报道:
Ann Arbor, MI, biotech Esperion Therapeutics ($ESPR) finally resolved the FDA snag standing in the way of its ambitious plans for a new cholesterol drug, clearing the company to begin late-stage trials with its potentially disruptive pill.
The agency has lifted a partial clinical hold that limited Esperion from running studies longer than 6 months, the company said. The FDA had previously expressed concern about the drug's toxicity, but, vetting preclinical data the biotech submitted late last year, regulators have given Esperion the go-ahead to keep moving.
Now the biotech plans to take its oral cholesterol fighter, ETC-1002, into Phase III later this year, CEO Tim Mayleben said. Esperion is planning to enroll about 4,000 patients in a late-stage study to confirm the treatment's effect on LDL cholesterol, marking the next step in Esperion's plan to shoulder in on the blockbuster race for next-generation cardio drugs.
ETC-1002 is a small-molecule therapy that has successfully slashed levels of LDL, or "bad," cholesterol in clinical trials. So, too, have the blockbuster-in-waiting injections from Amgen ($AMGN) and partners Sanofi ($SNY) and Regeneron ($REGN). But, unlike those likely expensive antibodies, Esperion's candidate is positioned as a cheaper, easier solution, Mayleben said at last month's JP Morgan Healthcare Conference.
As the CEO sees it, his company's pill will be an ideal second option for the millions of patients who either can't tolerate statins or can't get to healthy LDL levels on the generic drugs. The antibodies, which work by blocking the proteinPCSK9, will end up as third-line therapies, he said, subscribing to "the radical belief that patients will prefer an oral therapy over injection."
First Esperion will have to come through in Phase III, of course. Thanks to a nearly $100 million public offering late last year, the biotech has enough cash to go it alone, but Mayleben said Esperion may look to partner up with a larger drugmaker with experience running large cardiovascular trials.
Beyond ETC-1001, Esperion has only one other disclosed asset, a preclinical metabolic treatment. That's a reflection of what Mayleben said is an all-hands-on-deck devotion to the top prospect. Esperion is effectively a one-trick pony, he said, but that's "not so bad if you're riding Seabiscuit."
-
投融资
-
企业风采

-
市场商机

-
焦点事件

-
焦点事件

-
并购