Cytokine induced angiogenesis in chick embryos.
Objective:
The purpose of this experiment was to explore the effects of the growth factors bFGF and VEGF on blood vessel formation within the chorioallantoic membrane (CAM) of chick embryos.

Background Information:
Several growth factors are involved in the development of the blood system in both developing chick embryos and their yolk sacs (Gilbert, 2003). The process of blood formation, known as hematopoiesis, is divided into two categories: the embryonic stage and adult stage. This experiment investigates the embryonic stage of hematopoiesis, during which time blood vessels are created and circulation begins (Gilbert, 2003).
The construction of blood cells occurs through two processes, the first of which is vasculogenesis. During vasculogenes, "blood islands" appear in the yolk sac of the embryo and form capillary networks (Gilbert, 2003). Prior to the actual formation of these "blood islands," specified mesoderm cells must first form hemangioblasts, the precursors of blood cells and blood vessels (Gilbert, 2001 and 2003). Hemangioblasts give rise to both angioblasts or hematopoietic stem cells, which form endothelial cells and blood cells, respectively (Gilbert, 2003). These cells then condense to form blood islands, the inner most cells of which are composed of hematopoietic stem cells, and the outermost cells of angioblasts (which will eventially line the inside of the blood vessels) (Gilbert, 2000 and 2003). The endothelial cells will form tubes and connect to create a network of capillaries known as the primary capillary plexus (Gilbert, 2000). The second stage of blood formation in chick embryos, known as angiogensis, is marked by the the formation of a more complex vessel system. This is accomplished by a "remodeling" of established vessels to complete the circulatory system (Gilbert, 2000 and 2003).
Three paracrine growth factors (also known as cytokines) are involved in the process of vasculogeneis: basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and angiopoietin-1 (Ang1) (Gilbert, 2000 and 2003). Overall, these factors are concentrated by the extracellular matrix of the mesenchymal cells at the sites of hematopoiesis, and they contribute to blood cell and lymphocyte formation. On a smaller scale, the first growth factor, bFGF, is responisble for both the specification of mesoderm cells to form hemangioblasts, and the vascularization of the chorioallantoic membrane (CAM) tissue (Gilbert, 2000 and 2003). The CAM membrane is located beneath the shell membrane, and is formed by the fusing of the chick allantoic membrane with the mesodermal layer of the chorion (Gilbert, 2000 and 2003). It is this membrane that absorbs calcium from the eggshell, needed for the formation of the skeleton of the chick embryo (Gilbert, 2003). The second growth factor, VEGF, is secreted by the mesenchymal cells and instigates the formation of blood islands into blood vessels. Lastly, Ang1 mediates the interaction between the endothelial cells and the smooth muscle cells that cover the vessels (Gilbert, 2000)
In the first of two experiments, the VEGF and bFGF growth factors were used to induce angiogenesis; both are applied to the CAM membrane of the chick embryo. In the second experiment, only the effect of bFGF on blood vessel formation (or angiogenesis) within the CAM membrane was evaluated.
Procedure:
1.Cut small pieces of Whatmann 3MM filter paper (3mm X 3mm) and soak them with 3 ml Hydrocortison-acetate (3 mg/ml in ethanol) (Sigma). Air-dry the filter discs for 1.5 hrs.
2.Obtain 4mL each: bFGF (1.5 mg/ml), VEGF (1.5 mg/ml), or DMEM (a tissue culture medium in which the control filter discs should be soaked). Pour the solutions into separate petri dishes that are labeled with the names of the compounds. Soak the filter paper disks in the solutions for 30 minutes.
3.While the filter paper is soaking, obtain ten-day-old chick eggs and wash them in 70% ethanol. Label the eggs as either DMEM, bFGF, or VEGF using a pencil. Open the blunt end of the egg with forceps. Peel back the shell membrane, being careful not to damage the CAM membrane.

4.Drop the filter paper over the embryo in the area with the least number of visible blood vessels. Cover the hole in the shells with Scotch tape, and place in an incubator set at 37C (being careful not to jostle the embryos) for four days.
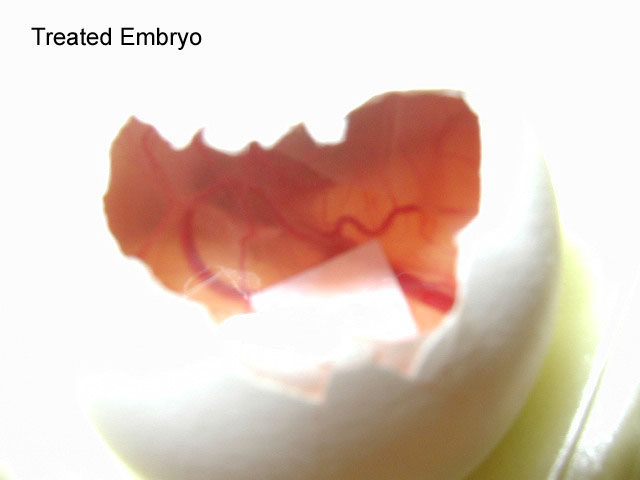
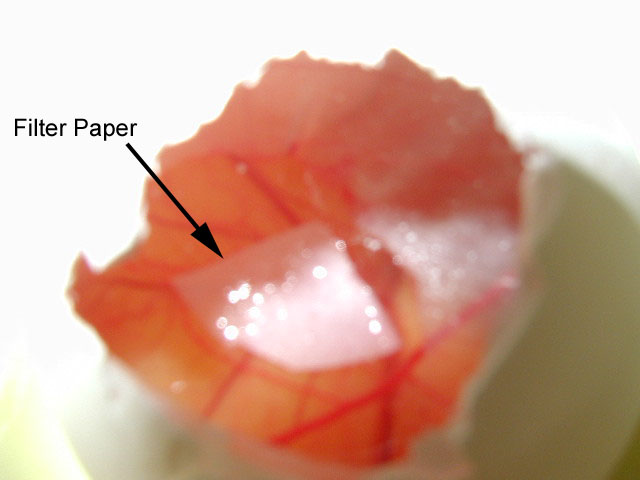
5.After the four-day period, remove the tape covering the holes and extract the filter paper using a pair of forceps. Examine the filter paper for the presence of blood vessels. Count all blood vessels on the filter paper and record the numbers within your lab notebook. Compare to the other conditions.
Results:
The embryos of the first experiment were treated with one of two growth factors, either bFGF or VEGF. Those treated with bFGF showed the greatest amount of blood vessel forma- tion when compared with the DMEM and VEGF embryos (pictures of the results). A problem may have occurred with the VEGF, as it had been in the lab for an extended period of time and may have been too old to produce accurate results.
Of the twelve embryos prepared for the second experiment (during which only the effects of bFGF on blood vessel formation was evaluated), seven survived. When opened, those embryos that did not survive had significantly darker amnionic fluid than their living counterparts. In addition, the amnionic fluid of the dead embryos appeared cloudy and gave off a distinct odor. Of the seven surviving embryos, only the filter paper disks of three were removed successfuly (with portions of the CAM membrane attached). In other cases, when removing the disks, blood vessels were cut in such a way as to contaminate the filter paper squares; some removed squares were dyed red by the ruptured blood vessels, and the CAM membrane was either dislodged or lost.
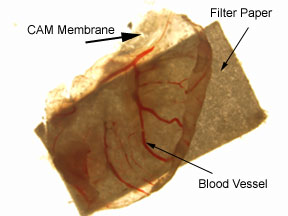
Figure 1. Photograph of CAM membrane attached to a filter paper square (3mm x 3mm) treated with DMEM. The CAM membrane was removed from a 15-day-old chick embry, after the membrane was exposed to a treated filter paper square for five days.
When examining the numbers of blood vessels on the CAM membranes treated with either DMEM or bFGF, the one treated with bFGF had a greater number of blood vessels (13) as compared with the vessels observed on the two disks treated with DMEM (7 and 12, respectively); however, it must be taken into account that only three test results were collected. In addition, a significant difference between the number of blood vessels of either the DMEM or bFGF CAM membranes did not exist.
Discussion:
From the first experiment, bFGF was shown to be influential in blood vessel formation. After embryos were exposed prematurely to the growth factor, a large number (in comparison with either the DMEM or VEGF blood vessel counts) of blood vessels formed. Insufficient data from the second experiment prevents any definite conclusions about the role of bFGF on blood vessel formation within the CAM membrane. However, the bFGF CAM membrane did exhibit more blood vessels than either of the DMEM segements. The bFGF membrane segment had a total of thirteen blood vessels, whereas the DMEM segments had seven and twelve blood vessels, respectively. In comparison to the bFGF blood vessel count (13), the average of the DMEM filter square blood vessels (7.5) was moderately lower.
The first experiment showed a strong correlation between the growth factor and increased blood vessel formation on the CAM membrane of chick embryos. bFGF, produced in the CAM membrane, is required for the process of vasculogenesis, during which time hemangioblasts are specified and blood vessels are created (Gilbert 2003). A greater concentration of bFGF may cause more blood vessels to appear during vasculogenesis. Within an area of high bFGF concentration, more mesoderm cells may be specified to form hemangioblasts, and more blood islands and vessels will be produced (Gilbert, 2003). In addition, the importance of these growth factors is demonstrated by mutant embryos that lack the proper receptors for bFGF (and also VEGF), which have a significantly lower chance of survival as vascular development will not occur properly.




















