The Effects of Ultraviolet Light on the Fertilization
Jill K. Flemming, Franklin & Marshall College, Class of 2001
Introduction
The objective of this project is to observe the effects of UV radiation on both fertilization and development in the sea urchin embryo.
Ultraviolet (UV) light is becoming an increasing concern in today's society. Humans, as well as other organisms, are being exposed to more and more UV rays due to the expanding hole in and thinning of Earth's ozone layer.
One way to examine the specific effects of the UV light on living organisms is to study the sea urchin. The embryo of the sea urchin is transparent, so it makes viewing development easier than most other organisms. The sea urchin spawns and develops in shallow tide pools. Therefore, the effect of UV light is a concern for fertilization and embryonic development of the sea urchin embryos. Previous experiments have shown that at a depth of one meter (which is the approximate depth of most tide pools) only 20% of UV radiation has been filtered out by the water, leaving 80% of the harmful rays to affect the urchin embryos. UV light disrupts microtubule and cytoskeleton development, as well as cleavage and gastrulation. Therefore, we expect to see the irradiated embryos dividing unevenly, cells cleaving improperly (partially or not at all), and gastrulation probably not occurring at all.
Animals
We will be using Lytechinus variegatus from Florida. The gametes and embryos can be kept at room temperature.

Figure 1. Lytechinus variegatus adults
Protocol
This experiment will involve the segregation of eggs into four samples. Sample 1 will be the control, exposed to NO radiation. In sample 2, the eggs will be irradiated before fertilization. Sample 3 will be irradiated 10 minutes after fertilization. Sample 4 will be irradiated 30 minutes after fertilization. The experiment is to be carried out as follows.
Collection of Gametes
Follow procedure given in basic protocol.
UV Radiation
Irradiate sample #2 (unfertilized eggs) using the UV crosslinker set on the default setting of intensity 120,000 microJoules/cm2 for a time of two minutes. The crosslinker has radiation with a wavelength of 250nm.
Fertilization
Fertilize all unirradiated samples as one batch, and then split into three separate test tubes following the completion of fertilization. Fertilize the irradiated sample as a separate batch. For fertilization procedure, follow instructions given in basic protocol.
UV Radiation(samples #3 & #4)
Irradiate sample #3 (10 minutes after fertilization) and #4 (30 minutes after fertilization). Use the same preset button on the UV crosslinker. Observe embryos and note differences in all three irradiated samples and the control. Embryos should be observed on the evening of the first day when fertilization takes place. Then observe once each day for the next three days. Record any differences from the control (tube #1).
Preparation of fixed embryos for immunocytochemistry(all samples)
1. Transfer 50 ml of your embryo cultures to centrifuge tubes. Spin at mark 4 for 5 minutes. Check that you can see a pellet of embryos at the bottom. Quicklypour off the artificial sea water (ASW). Try to remove as much as possible , but don't worry about a little ASW left in the tube.
2. Gently swirl tube to resuspend the embryos. Add 40mL ice cold methanol and allow to fix on ice for no more than 20 min. By this time, the embryos should have settled to the bottom of the tube.
3. Decant off the methanol and resuspend the embryos about 25 ml ice cold ASW. ON ICE, let the embryos settle to the bottom of the tube by gravity.
4. Decant off the ASW and resuspend the embryos in fresh ice cold ASW. Store in the refrigerator until needed. Embryos will settle.
5. Decant ASW, swirl to resuspend in remaining ASW. Transfer to four 1.5 ml microfuge tubes.
Immunofluorescent staining of archenteron cells(all samples)
1. Allow 2 tubes of embryos to settle for 10 minutes.
Gently remove most of the liquid.
2. Add 200 ml 5C7 antibody (stains vegetal plate and archenteron) to one tube and 200 ml 10%
normal goat serum to the other. Let sit for 45 minutes at room temperature. Embryos will
settle.
3. Remove most of liquid. Add 1 ml ASW to wash. Allow to settle, remove liquid. 4. Add 200 ml
Fluorescein-congugated Goat anti-mouse IgG to both tubes. Let sit for 45 minutes at room
temperature. Embryos will settle.
5. Remove most of liquid. Add 1 ml ASW to wash., Allow to settle, remove liquid. Add 100 ml
PBS.
6. Transfer 10 ml of each sample to microscope slides. Check that there are embryos.
Coverslip. Examine using epifluorescence.
7. Observe differences from control.
Results
Throughout the course of our experiment, we observed our embryos at five different times. The first observation was made only 30 minutesafter fertilization. The only sample that was not observed at this time was sample #4 because it was only irradiated 30 minutes after fertilization. At this 30 minute mark, we saw normal development in the control. Cells were already beginning to undergo the first cleavage. The other two irradiated samples had not yet begun to cleave at this point.
After 5 hours, the control sample showed normal development at the blastula stage. Sample 2 exhibited a huge variance in stage. Some were halted after first cleavage, while others had divided several times. Most cells that did divide, had done so unevenly. Sample 3 exhibited many unusually small cleavages. Sample 4 contained cells that appeared to have divided anywhere from two to six times. The divisions in this sample were extremely irregular.
On the second day after fertilization, the control sample was a normally developing embryo at the archenteron stage. Samples 2, 3, and 4 all looked very similar. They were irregularly dividing, had variances in numbers of cell divisions and shapes of cells, and had no archenterons.
The third day observations exhibited controls which were at the pluteus larvae stage. The larvae were normal and mobile. The other three samples all looked similar. They were very irregular and underdeveloped. Sample 2 had an especially large number of dead cells that appeared in clumps.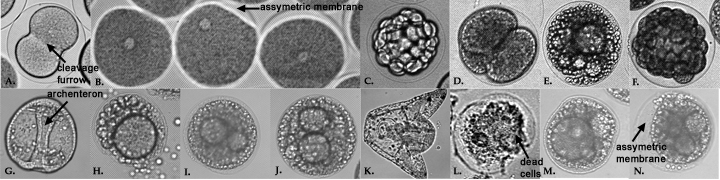
Figure 2.
A, C, G, & K are Lytechinus variegatus controls at the following stages after fertilization: A=30 minutes, C=5 hours, G=2 days, and K=3 days.
D, H, & L are embryos which have been irradiated just prior to fertilization. Pictures were taken at D=5 hours, H=2 days, and L=3 days.
B, E, I, & M are images of embryos which have been irradiated 10 minutes after fertilization. Pictures were taken at B=30 minutes, E=5 hours, I=2 days, and M=3 days.
F, J, & N are embryos which have been irradiated 30 minutes after fertilization. Pictures were taken at F=5 hours, J=2 days, and N=3 days.
After fixing and staining all samples with fluorescent 5C7 at the two-day old stage, the archenterons and vegetal plates could only be observed in control embryos. The other samples showed no archenteron or vegetal plates staining. Several small clumps of fluorescent staining could be observed, however they were free floating and not located where the archenteron or vegetal plate should be.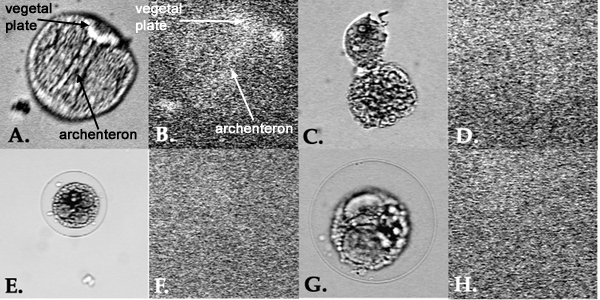
Figure 3.
A. Control Lytechinus variegatus at 5 days after fertilization.
B. Fluorescent staining of control embryo from image A. The archenteron and vegetal plate are both visible.
C. L. variegatus embryo from sample 2, which was UV irradiated just prior to fertilization.
D. Fluorescent staining of same embryo from image C. No archenteron or vegetal plate visible.
E. L. variegatus embryo from sample 3, which was UV irradiated 10 minutes post-fertilization.
F. Fluorescent staining of same embryo from image E. No archenteron or vegetal plate visible.
G. L. variegatus embryo from sample 4, which was UV irradiated 30 minutes post-fertilization.
H. Fluorescent staining of same embryo from image G. No archenteron or vegetal plate visible.
Discussion
Our results were as expected. Since UV radiation inhibits cytoskeletal function, we observed improper cell divisions, and no gut formation in all irradiated embryos. Control samples looked normal, confirming that our experiment was performed correctly. No significant differences could be observed between samples 2, 3 and 4, where irradiation took place at different times. For this reason, we conclude that the time of irradiation, before or after fertilization, does not make a difference in the overall effect on the embryos. Irradiation at any time does not allow embryos to develop normally since it disrupts cytoskeletal function, which is crucial to proper embryonic development.




















