The effects ofdifferent concentrations oflithium chloride on thedevelopment
Abstract
Previous studies by Stachel and colleagues indicate that lithium chloride induction of post-midblastular Brachydanio rerio embryos results in deficiencies in normal anterior-posterior development. To determine whether or not higher concentrations of lithium chloride lead to successively greater defects in anterior-posterior development, D. rerio embryos were induced with the following variable concentrations of lithium chloride: 0.45 M, 0.30 M, 0.15 M, and 0.00 M. Embryos induced with 0.15M of LiCl exhibited little or no phenotypic deviation from the control group, whereas 0.30 M and 0.45 M LiCl-induced embryos did not develop eyes and showed signs of perturbed tail development.The results of this experiment suggest that the degree of inhibition of normal anterior-posterior development increased in embryos induced with higher concentrations of lithium chloride. Lithium chloride induction may be responsible for the reduced transcription of goosecoid in the anterior regions of D. rerio or the prevention of cell movements allowing the anterolateral accumulation of goosecoid, resulting in the perturbation of normal anterior development (Stachel et. al., 1993).
Introduction
Lithium chloride is a known teratogen which alters development in a variety of organisms including sea urchins, Xenopus (frogs), andBrachydanio rerio (Zebrafish) (Gilbert, 2003). In sea urchin embryos, lithium chloride causes the accumulation of nuclear Beta-catenin in every cell, and transforms presumptive ectoderm into endoderm (Gilbert, 2003). Lithium exposure in cleavage-stage embryos ofXenopus inhibits dorsal/ventral axis specification and results in radially-symmetric, dorsal-anteriorized embryos (Stachel et. al., 2003). The research conducted by Stachel and colleagues suggests that lithium induction of pre-midblastular Zebrafish prevents normal dorsal/ventral axis patterning by acting as an inhibitor to the phosphoinositol pathway, which results in goosecoid and nogginexpression outside the region of the presumptive embryonic shield instead of these genes being confined to the region proximal to the dorsal blastopore lip.
Experiments by Stachel and colleagues have shown that the development of anterior structures is dependent on Wnt signaling, especially the transcription of goosecoid, which codes for a dorsalizing protein necessary for normal anterior development (Stachel et. al., 2003). The increase in gene expression of organizer-specific proteins such as Goosecoid in the presumptive ventral regions of the organism produces different phenotypic results when the induction occurs at certain stages in development (Stachel et. al., 1993). For instance, the exposure of embryos to LiCl before the midblastular transition (2 hour stage) results in hyperdorsalization and the inhibition of normal dorsal/ventral axis patterning (Deitrich, 1999). In contrast, embryos exposed to LiCl at the four-hour stage after the midblastular transition experienced normal dorsal/ventral axis specification but perturbed development of anterior structures such as eyes (Stachel et. al., 1993).
The purpose of this experiment is to study the effects of lithium teratogenesis on post-midblastular embryo to observe whether or not the inhibition of anterior development occurs along a gradient, with increasing concentrations of lithium chloride leading to incrementally more severe defects in anterior development. Alternatively, there may be a certain threshold at which lithium induces defects in anterior development. Indications of the former hypothesis would consist of lithium teratogenesis leading to, for example, partial formation of an eye at one concentration and the complete absence of eye formation at a slightly higher concentration, then it may be possible that lithium teratogenesis affects development along a gradient. In accordance with the latter situation, the observable effects of lithium teratogenesis would consist in the complete absence of the eyes at one concentration and complete formation and presence of all eye structures including retina and lens at a slightly lower concentration.
Objective
The purpose of this experiment is to monitor the effects of different concentrations of LiCl on anterior development in zebrafish embryos. This will be carried out by assessing the degree to which certain amounts of the lithium cholride teratogen affect the morphology of anterior structures such as the eye.
Materials
60-80 Brachydanio rerio embryos at the sphere/dome-stage of development (see procedure for more details)
LiCl (0.15 M, 0.30 M, and 0.45 M) in Zebrafish embryo medium
distilled H2O
9 60 mm glass petri dishes
wide-mouth pastuer pipette (with large enough opening for suctioning chorionated zebrafish eggs)
dissection scope
Procedure
1. Obtain 15-20 zebrafish embryos at the sphere/dome-stage of development (four hours post-fertilization) for each of the four titrations of lithium chloride to be tested, inclusive of the control group. Therefore, about 60-80 sphere/dome-stage embryos must be isolated in total. For a description of sphere/dome-stage embryos, refer to the Zebrafish Information Network homepage (www.zfin.org).
2. Place the embryos into four separate petri dishes, each containing 10 mL of Zebrafish embryo medium.
3. Next, procure three dishes and fill them with the following concentrations of LiCl:
0.45 M
0.30 M
0.15 M
A table including the calculations for the three LiCl titrations used in the original experiment is included in the Materials and Methods section of this page.
4. Transfer each population of embryos from the embryo medium to the LiCl-containing dishes using a severed pipette and immerse them in solution for 10 minutes.
5. After 10 minutes, rinse the embryos by placing each group in a separate dish containing 10 milliliters of embryo medium, and then transfer back to the original embryo-medium-containing dishes. Be sure to label the dishes with the corresponding amount of LiCl to which they were induced.
6. Photograph the embryos after 24 hours to observe anterior development, carefully noting deviations in anterior patterning and formation from the control population.
Materials and Methods
The embryos used in this experiment were at the sphere stage of development, which is equivalent to approximately four hours of incubation post-fertilization. More importantly, the sphere stage occurs after the midblastular transition, whereupon the embryo begins to translate its own genes, cell movements occur, and a pattern of slow, asynchronous cleavage begins (Gilbert, 2003). The exposure of each of the variant groups to lithium chloride consisted of a ten-minute incubation period for each so that the time of incubation would remain concurrent with the sphere stage and not extend beyond this period of development. Sphere-stage embryos were isolated according to Stages of Embryonic Development of the Zebrafish (Zebrafish Information Network, 1995). Isolated embryos were collected in Zebrafish Embryo Medium, which was prepared according to the developmental biology lab website (DB Lab, 2003). During the induction perion, approximately 10-15 Brachidanio rerio embryos were treated with the following titrations of LiCl: 0.45 M, 0.30 M, and 0.15 M made up in Zebrafish Embryo Medium. The calculations for the concentrations of Lithium Chloride that were used are as indicated in Table I:
Table I: Variable Molarity of Lithium Chloride Titrations
| Formula weight | Experimental concentration (M) | Conversion factor | Amount LiCl added |
| 42.39 g/ 1 mole | 0.45 M/ L | .01 L/ 10 mL | 0.19 g/ 10 mL |
| 42.39 g/ 1 mole | 0.30 M/ L | .01 L/ 10 mL | 0.13 g/10 mL |
| 42.39 g/ 1 mole | 0.15 M/ L | .01 L/ 10 mL | 0.06 g/ 10 mL |
The fourth group did not contain any LiCl and served as a control, thus no calculations were required for this titration. Subsequent to the ten-minute incubation period of the three variable groups in the LiCl solutions, all embryos were rinsed with Zebrafish embryo medium and incubated overnight at 28°C.
Results
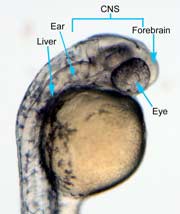
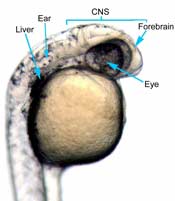
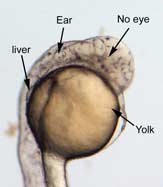
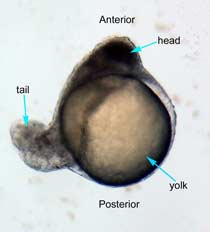
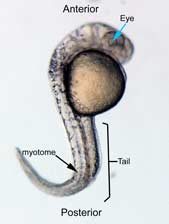
In the control group embryos, anterior-posterior patterning was normal, with full formation of the terminus structures, the retina, and the lens of the eye (Figure 1A). In embryos treated with 0.15 M LiCl, the eyes appear to have developed normally, but the eye shown in Figure 1B is relatively closer to the anterior terminus than the eye in Figure 1A. The 0.15 M LiCl-treated embryos exibited slightly stunted growth at their anterior and posterior termini; the tails were also slightly bent and rounded at the tip (Figure 2B. The 0.30 M LiCl-treated embryos did not develop eyes, and exhibited slight curvature of the dorsal region (Figure 1C). These embryos exhibited even less development of the anterior and posterior termini than the 0.15 M embryos, with stubby tails and large yolk sacs (Figure 2C).
All embryos in Figure1 exhibit development of ears, liver, and the CNS. The average survival rate for embryos in the 0.00 M, 0.15 M, and 0.30 M solutions were 19/19, 18/20, and 15/21 respectively. The 0.45 M LiCl -treated embryos had lower viability, with only seven of the original nineteen embryos surviving the 24-hour period from the time of incubation to the time of data collection. The resulting embryos were fragile, and some did not survive the dechorionation process and their cells became disaggregated by the pull of the forceps and the influx of Zebrafish embryo medium into the opened chorion. This group displayed a lack of anterior-posterior development, with no definable head structures, and stunted tail development (Figure 2D).
|
|
|
A 0.00 M LiCl | B 0.15 M LiCl | C 0.30 M LiCl |
Figure 1 (A) Control embryo, typical anterior/posterior patterning with fully-formed anterior structures including the eye, forebrain, CNS, ear and liver (B) Embryo induced with 0.15 M LiCl shows liver, ear, eye, forebrain, and CNS development (C) 0.30 M LiCl-induced embryo shows no eye formation, though ear and liver are present.
 | 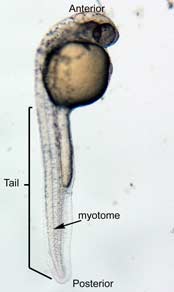 |
A 0.00 M LiCl | B 0.15 M LiCl |
|
|
C 0.30 M LiCl | D 0.45 M LiCl |
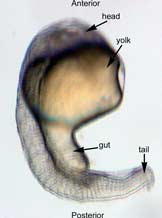
Figure 2 Photographs taken 26 hours after LiCl induction (A) Control embryo shows normal tail formation (B) Embryo shows normal tail development (C) Tail appears stunted and crooked, gut is shortened, head is smaller, and yolk is enlarged and misshapen (D) Head and tail are stunted, anterior structures are missing, yolk is enlarged.
Discussion
These results demonstrate that lithium chloride treatment affected the anterior-posterior morphogenesis of Brachydanio rerio embryos such that higher concentrations of this substance lead to increasingly greater inhibition of anterior and posterior development. The 0.15 M LiCl-treated embryo were missing their anteriormost structures. The embryos treated with 0.30 M LiCl exhibited greater teratogenic effects on the development of anterior and posterior terminus structures. Higher concentrations of LiCl also led to more morphological defects in both the anterior and posterior terminal regions, further confirming the hypothesis that the concentrations of LiCl teratogen is directly related to the phenotypic effect on Zebrafish.
Lithium-induced teratogenesis has been shown to perturb the development of ventral structures in premidblastular Zebrafish, when the blastula is still forming morphogenetic gradients of proteins in the Wnt signaling pathway (such as b-catenin) and continuing to transcribe maternal mRNAs. In these early embryos, lithium teratogenesis leads to the expression of bustled and radialized phenotypes, which exhibit hyperdorsalization (Stachel et. al., 1993). In the sphere-stage embryos, morphogenetic gradients have already been established, so the effect of lithium in this case probably occurs downstream of these events.
Studies conducted by Stewart and Gerhart (1990) showed that lithium inhibits gastrulation movements in Xenopus, resulting in incomplete involution and thus incomplete induction of anterior structures (Stewart and Gerhart, 1990). This could possibly indicate that lithium teratogenesis perturbs development in later embryos by preventing gastrulation.However, the phenotype of lithium-treated Zebrafish embryos does not appear to be equivalent to that of Xenopus embryos. The most striking effect is on the normal formation of structures anterior to the eye. Stachel and coworkers (1993) have presented evidence of the normal development of central nervous system structures anterior to the presumptive eye region when lens and retina develop abnormally. This suggests that the incomplete gastrulation model of lithium teratogenesis in Xenopus does not fully explain the phenotypic effects of lithium in Brachydanio rerio embryos. This evidence also suggests that there may be differential signaling patterns involved in Xenopus as compared to Zebrafish eye formation.
Lithium dorsalizes sea urchin embryos by increasing the levels of b-catenin throughout the cytoplasm, which leads to the radialization ofgoosecoid expression and subsequent augmentation of organized mesoderm throughout the marginal zone and into the presumptive ventral regions of the embryo (Gilbert, 2003). The embryos in this experiment did not show a great degree of dorsalization, except possibly the 0.45 M LiCl embryo, but further tests would need to be conducted before determining whether or not lithium induces dorsal specification of tissues.
Fortunately, such studies have been conducted by Stachel and colleagues (1993). They used a fluorescent market gene intercalated in the genome adjacent to the goosecoid transcript, so that the marker appears in the wherever goosecoid protein is present. The results of their experiment on lithium induction in Zebrafish shows that the distribution of goosecoid transcripts increases substantially ten hours after development. During this period, rostral crescent swelling and medial strip patterns usually exhibiting goosecoid transcripts appear normal. However, the lateral wings, areas of goosecoid expression found in normal embryos, are missing, which suggests that late-lithium exposure either reduces transcription of goosecoid in the lateral wing region or prevents cell movements directing the anterolateral accumulation of goosecoid (Stachel et. al., 1993). This reduction in the expression of goosecoid in the anterolateral region suggests that lithium is partially responsible for the inhibition of normal anterior development.
Acknowledgements
I would like to recognize several individuals for making this experiment possible. Thank you first of all to my lab group members, Kristi La Salle, Brandt Rakowski, Katy Lewis, and Erin Betters for isolating the sphere-stage Zebrafish embryos used in this experiment. I would like to recognize Peter Burgess of Franklin and Marshall College who conducted a similar experiment on Medaka embryos that inspired me to conduct this experiment. Thanks to the lab personnel responsible for feeding the fish, and to Professor Judy Cebra-Thomas and Fraser Tan for preparing the LiCl concentrations used in this experiment. Thanks to Scott Stachel and his colleagues for providing useful information on previous experiments in lithium induction of Zebrafish and to Scott Gilbert for providing a wonderful resource for being able to interpret the data collected in this experiment. Additionally, I would like to thank William Gresh for advising me in the writing of this lab report.