alamarBlue® Cell Viability Assay Protocol
实验概要
Cell health can be monitored by numerous methods. Plasma membrane integrity, DNA synthesis, DNA content, enzyme activity, presence of ATP, and cellular reducing conditions are known indicators of cell viability and cell death. alamarBlue® cell viability reagent functions as a cell health indicator by using the reducing power of living cells to quantitatively measure the proliferation of various human and animal cell lines, bacteria, plant, and fungi allowing you to establish relative cytotoxicity of agents within various chemical classes. When cells are alive they maintain a reducing environment within the cytosol of the cell. Resazurin, the active ingredient of alamarBlue® reagent, is a non-toxic, cell permeable compound that is blue in color and virtually non-fluorescent. Upon entering cells, resazurin is reduced to resorufin, a compound that is red in color and highly fluorescent. Viable cells continuously convert resazurin to resorufin, increasing the overall fluorescence and color of the media surrounding cells.
alamarBlue® cell viability reagent is used to assess cell viability by simply adding the 10X, ready-to-use solution to mammalian or bacterial cells in culture media (Figure 1). There is no requirement to aspirate media from cells or place cells in minimal media. Consequently, alamarBlue® reagent can easily be used in a single tube or microtiter plate format in a “nowash” fashion. Simply add alamarBlue® reagent as 10% of the sample volume (i.e., add 10 μL alamarBlue® reagent to 100 μL sample), followed by a 1–4 hours incubation at 37ºC. Longer incubation times may be used for greater sensitivity without compromising cell health (Figure 3 and see Frequently Asked Questions). The resulting fluorescence is read on a plate reader or fluorescence spectrophotometer. Alternatively, the absorbance of alamarBlue® reagent can be read on a spectrophotometer. Finally, results are analyzed by plotting fluorescence intensity (or absorbance) versus compound concentration.

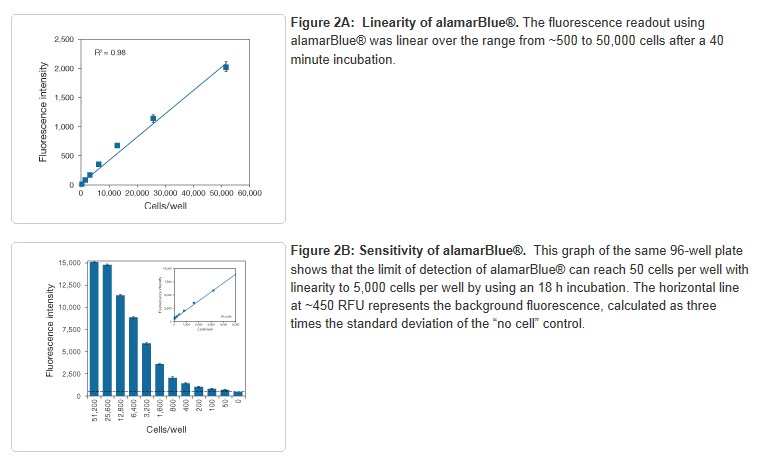
alamarBlue® reagent can detect as few as 50 cells per well in a 96-well plate. To evaluate the sensitivity of alamarBlue® reagent, a serial dilution of HUVEC cells was performed in a black, clear-bottom 96-well plate. Cells were then treated with alamarBlue® reagent and the fluorescence was measured 40 minutes and 18 hours later. After 40 minutes, the fluorescence intensity of alamarBlue® reagent was directly proportional to cell number in the range of 500–50,000 cells. After 18 hours, the fluorescence intensity of alamarBlue® reagent was directly proportional to cell number in the range of 50–5,000 cells, leading to more sensitive detection (Figure 3).

实验材料
Materials required but not provided
1. Mammalian or bacterial cells in appropriate medium
2. Appropriate 96- or 384-well plates
3. Optional: 3% SDS in phosphate buffered saline (PBS), pH 7.4
实验步骤
Optional: Treat cells with the test compound 24–72 hours prior to performing the alamarBlue® cytotoxicity assay.
1. Add 1/10th volume of alamarBlue® reagent directly to cells in culture medium as described in Table 1.
Format | Volume of cells medium | Volume of 10x alamarBlue to add |
Cuvette | 1 mL | 100 µL |
96-well plate | 100 µL | 10 µL |
384-well plate | 40 µL | 4 µL |
2. Incubate for 1 to 4 hours at 37°C in a cell culture incubator, protected from direct light.
Note: Sensitivity of detection increases with longer incubation times. For samples with fewer cells, use longer incubation times of up to 24 hours.
3. Record results using fluorescence or absorbance as follows:
Fluorescence: Read fluorescence using a fluorescence excitation wavelength of 540–570 nm (peak excitation is 570 nm). Read fluorescence emission at 580–610 nm (peak emission is 585 nm).
Absorbance: Monitor the absorbance of alamarBlue® at 570 nm, using 600 nm as a reference wavelength (normalized to the 600 nm value).
Note: Fluorescence mode measurements are more sensitive. When fluorescence instrumentation is unavailable, monitor the absorbance of alamarBlue® reagent. Assay plates or tubes can be wrapped in foil, stored at 4°C, and read within 1–3 days without affecting the fluorescence or absorbance values.
4. Optional: Add 50 μL 3% SDS directly to 100 μL of cells in alamarBlue® reagent to stop the reaction.
注意事项
1. General Questions
Q: How does alamarBlue® work?
A: Healthy living cells maintain a reducing state within their cytosol. This “reducing potential” of cells converts alamarBlue® reagent into a detectable fluorescent (or absorbent) product.
Q: Is alamarBlue® reagent toxic?
A: No. alamarBlue® reagent is a safe, non-toxic reagent to both the sample and user.
Q: Does alamarBlue® reagent need reconstitution?
A: No, alamarBlue® reagent is supplied as a 10X, ready-to-use solution.
Q: Can I use alamarBlue® reagent with suspension cells too?
A: Yes. alamarBlue® reagent works on adherent and suspension mammalian cells.
Q: Can I use alamarBlue® reagent with non-mammalian cells, such as bacteria?
A: Yes, alamarBlue® reagent has been shown to work with bacterial2 and plant cells.4
Q: alamarBlue® reagent is not the most expensive cytotoxicity indicator on the market, does that mean it doesn’t work as well as other reagents?
A: Actually, alamarBlue® reagent is comparable to other often more expensive cytotoxicity indicators.
Q: Since alamarBlue® is an absorbance or fluorescence readout, is it as sensitive as a luminescence product?
A: alamarBlue® reagent is sensitive enough to detect less than 50 mammalian cells in a single well of a 96-well plate.
2. Storage Questions
Q: What if I left the alamarBlue® stock reagent at room temperature, overnight?
A: The reagent is stable for up to 12 months when stored at room temperature (~22ºC).
Q: I accidentally froze the alamarBlue® stock reagent, can I still use it?
A: Yes. alamarBlue® reagent is stable to multiple freeze/thaw cycles. Be sure to heat the reagent in a 37°C water bath and mix the reagent to ensure a homogenous solution before use.
Q: Do I need to protect alamarBlue® reagent from light?
A: Yes, alamarBlue® reagent is very slowly converted into a fluorescent product over time, when exposed to light, thus leading to high background values. Store the reagent, protected from light.
3. Methods Questions
Q: What is the optimal incubation time and temperature of cells with alamarBlue® reagent?
A: Incubate the cells with alamarBlue® reagent for 1–4 hours at 37°C. For more sensitive detection with low cell numbers, increase the incubation time for up to 24 hours.
Q: Can you incubate cells with alamarBlue® reagent overnight?
A: Yes. However, signals from higher cell density samples may have “saturated,” which means the linearity of reagent may have reached a plateau. If this occurs, decrease the incubation time.
Q: What if I don’t have an instrument suitable for reading fluorescence?
A: The absorbance of alamarBlue® reagent also changes depending on cell viability and proliferation. Therefore, simply monitor the absorbance of the reagent at 570 nm, while using 600 nm as a reference wavelength.
Q: Is alamarBlue® assay strictly an endpoint assay?
A: No. While alamarBlue® can be used as a terminal readout of a population of cells, the reagent can also be used to continuously monitor cell viability and proliferation in real time. Since alamarBlue® reagent is non-toxic, you can incubate cells with reagent and monitor fluorescence (or absorbance) over time on the same sample.
4. Troubleshooting Questions
Q: What is the problem for observing high background fluorescence values?
A: The reagent may be breaking down due to exposure to light. Be sure to store alamarBlue® reagent in the dark and do not expose the reagent to direct light for long periods of time.
Q: Why are the fluorescence values so low in intensity?
A: Try increasing the incubation time of cells with alamarBlue® reagent, changing the instrument’s “gain” setting, and checking the instrument filter/wavelength settings. Make sure to have positive controls (living cells) in the experimental design for troubleshooting.
Q: Why are the fluorescence values so high that they are beyond the linear range of the instrument?
A: Try decreasing the incubation time or reducing the number of cells used in the experiment.
附 件 (共2个附件,占123KB)
2.jpg
60KB 查看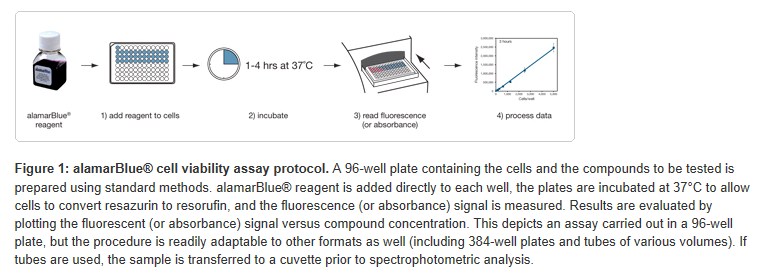
1.jpg
63KB 查看











