PNAS: TRIC逆离子通道在钙释放中活性的结构基础

2019年,中国科学院遗传与发育生物学研究所、哥伦比亚大学、纽约大学、西安交通大学、布鲁克海文国家实验室等单位合作在《美国科学院院报(PNAS)》期刊上发表了题为“TRIC逆离子通道在钙释放中活性的结构基础”的研究论文。
释放细胞内储存的钙对于许多细胞过程是至关重要的,包括肌肉中的刺激-收缩耦合。 三聚体胞内阳离子通道(TRIC)是ER / SR膜必不可少的组分,但它们的生理作用目前还不清楚。该项研究阐明了TRIC通道促进钙释放的逆离子机制。通过IP3或RYR受体释放期间的钙消耗使ER / SR膜电位去极化,从而阻碍钙的持续释放。 研究团队发现Ca2+与TRIC通道结合可稳定非导电构象;然而,在钙离子释放过程中产生的不含Ca2+的TRIC可以通过释放电压感应螺旋上的门控残留物,从而去除膜去极化造成的阻碍。产生的K +反离子电流可以恢复膜电位极化以持续释放钙。
研究还通过脂质组学技术发现脂质分子DAG与TRIC通道结合,揭示了细胞脂质信号与Ca2 +稳态之间的联系。在PIP2结合的CeTRIC中,PIP2的头部基团突出到孔中,并且肌醇环通过氢键结合到高度保守的残基(TM4上的K129 / R133),从而阻塞孔。磷酯酰肌醇特异性磷酯酶催化水解PIP2是DAG的主要来源之一。 DAG缺乏PIP2的肌醇环结构,这使得孔未在GgTRIC-A中被阻断(图2的C和D)。将结合PIP2的CeTRIC-2和结合DAG的GgTRIC-A叠加比较发现TM5b片段是结合不同脂质的构象开关。W173残基的吲哚环在结合DAG的GgTRIC-A通道的细胞质侧的三重轴上紧密结合,而在结合PIP2的CeTRIC-2中摆动(图2的E和F)。总之,研究数据表明DAG在成孔螺旋TM2和TM5的构象控制中起着关键作用,从而协同耦合通道门控。
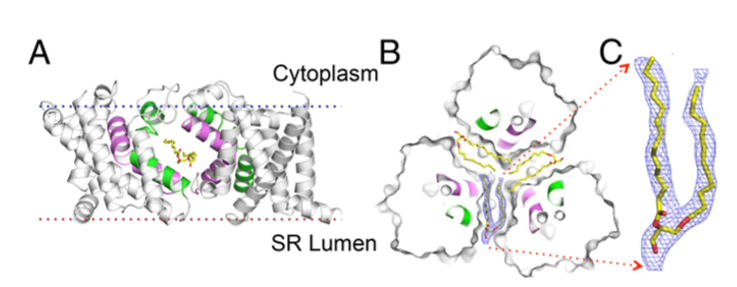
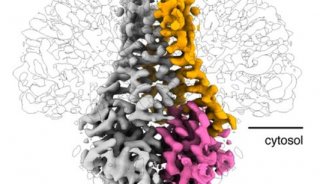
图1. Llipid binding in the lateral fenestration. (A) Ribbon drawing of the GgTRIC-A, with one protomer removed. The TM2 and TM5 helices from two protomers are colored purple and green, respectively. The lipid molecules within the lateral fenestrations are shown as stick, with carbon, nitrogen, and oxygen atoms colored yellow, blue, and red, respectively. (B) Cross-section view of GgTRIC-A. Three DAG molecules were superimposed into the lateral fenestrations, with one lipid covered by the 2Fo-Fc electron density map, contoured at 2.0σ, and colored as inA.( C) Close-up view of the lipid molecule, as in B.

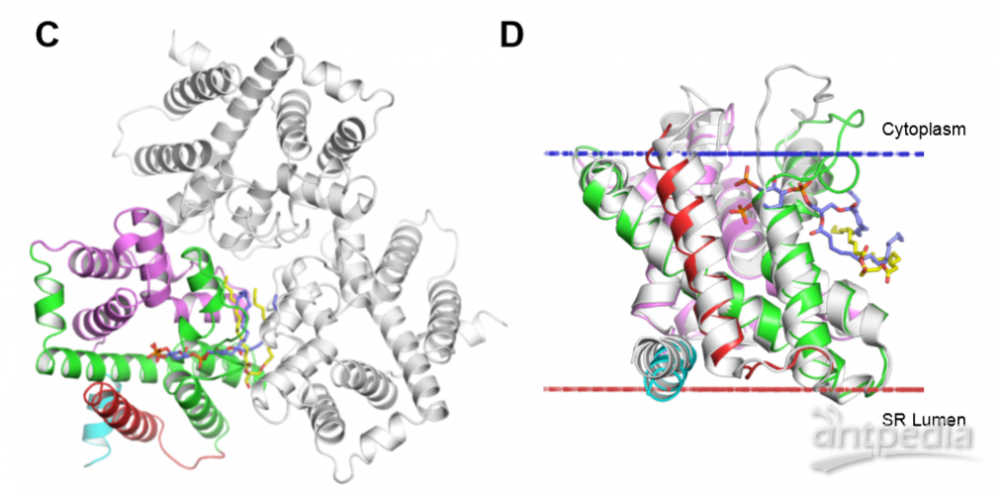

图2. Comparison of lipid binding in TRICs
(A) Lipidomics analysis of GgTRIC-A crystals. The lipid components in GgTRIC-A crystals are shown in the table and the heat-map (buffer as control). The enrichment of DAG is highlighted, as seen red in the table and black in the heat-map. LPS, lipopolysaccharides; PE, phosphatidylethanolamines; PS, phosphatidylserines; SM, sphingomyelins; PG, phosphatidylglycerols; PC, phosphatidylcholines; LPC, lysophosphatidylcholine; Cer, ceramide; CE, cholesteryl esters; Cho, cholesterols, DAG, diacylglycerol; TAG, triacylglycerol. (B) Variants of DAGs are detected in the crystals. The most abundant DAG candidates are highlighted in red. (C) Comparison of lipid binding mode in TRICs. Ribbon drawing of the GgTRIC-A trimmer, with one protomer coloured as in Fig. S2d. The DAG molecules are shown as stick, and colored in yellow. Whereas the PIP2 molecules observed in CeTRIC-2 was modeled into the GgTRIC-A through superimposed of TRICs structural model, and colored in blue. (D) Superimposition of DAG-bound GgTRIC-A and PIP2-bound CeTRIC-2. Ribbon drawings are shown for both GgTRIC-A and CeTRIC-2, with GgTRIC-A coloured as in (C), and CeTRIC-2 in grey. The DAG and PIP2 molecules are shown, as in (C). (E) Close-up views of the conformation of conserved W173 in the GgTRIC-A trimer. (F) Close-up views of the conformation of conserved W181 in the CeTRIC-2 trimer.