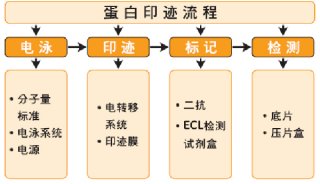
Western Blotting Protocols
back to top
Protocol
Standard vs. Rapid Immunodetection Procedures
There are two types of protocols for immunodetection: Standard and rapid.
Standard vs. Rapid Immunodetection
| Step | Standard Immunodetection | Rapid Immunodetection |
| Block the membrane | 1 hr | None |
| Incubate with primary antibody | 1 hr | 1 hr |
| Wash the membrane | 3 x 10 min | 3 x 5 min |
| Incubate with second antibody | 1 hr | 30 min |
| Wash the membrane | 3 x 10 min | 3 x 5 min |
| Add substrate | 5 min | 5 min |
| Total time | 4 hr 5 min | 2 hr 5 min |
Standard Immunodetection Methods Include the Following Steps:
Blocking unoccupied membrane sites to prevent nonspecific binding of antibodies
Incubating the membrane with primary antibody, which binds to the protein of interest
Washing to remove any unbound primary antibody
Incubating the membrane with a conjugated secondary antibody, which binds the first antibody
Washing to remove any unbound secondary antibody
Incubating the membrane with a substrate that reacts with the conjugated secondary antibody to reveal the location of the protein
Rapid immunodetection eliminates the blocking step and reduces the time necessary for the washing and incubation steps. The rapid immunodetection method works well to quickly visualize higher abundance proteins. Standard immunodetection, however, offers higher sensitivity and requires less optimization for new sample types.
back to top
Standard Immunodetection Method
Application
Standard immunodetection is performed on blotted proteins directly after electrotransfer. (If the membrane was dried after transfer, thoroughly wet the blot for 1 minute in methanol if using PVDF or Milli-Q water if using nitrocellulose before proceeding to immunodetection.)
The unoccupied membrane binding sites on the wet blot are blocked with optimized reagents. The drawbacks of this method are the need for blocking and the total time requirement of over 4 hours. The advantage is that standard immunodetection may require less optimization for new sample types.
Required Solutions
Primary antibody (specific for protein of interest)
Secondary antibody (specific for primary antibody), labeled with alkaline phosphatase or horseradish peroxidase.
Substrate appropriate to the enzyme conjugate (HRP or AP).
Appropriate wash buffer: Phosphate-buffered saline (PBST): 10 mM sodium phosphate, pH 7.2, 0.9% (w/v) NaCl, up to 0.1% Tween-20 detergent, TBST: 10 mM Tris, pH 7.4, 0.9% (w/v) NaCl, up to 0.1% Tween-20.
Blocking solutions: bovine serum albumin (BSA), 0.2%–5% (w/v), Tween-20,(0.05–0.1%), non fat dry milk (0.5–5%), Casein(1%).
Milli-Q water
Required Equipment
Shallow trays, large enough to hold blot
Glass plates
Plastic wrap (e.g., Saran™ film), freezer bag, or sheet protector
Autoradiography film and cassette
Dark room
Autoradiography film processing equipment
Set Up
Dilute the primary antibody in the blocking solution to the desired working concentration.
Dilute the secondary antibody in the blocking solution to the desired working concentration.
Note: Enough solution should be prepared to allow for 0.1 mL of antibody solution (primary and secondary) per cm2 of membrane.
back to top
Protocol
Perform SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transfer the protein to the membrane (electroblotting)
Wash the membrane twice with distilled water. If desired, stain the membrane with Ponceau Red solution for 5 minutes to visualize protein bands. (Stock solution: 2% Ponceau S in 30% trichloroacetic acid and 30% sulfosalicylic acid; dilute 1:10 for use.) Rinse the membrane in water until protein bands are distinct and mark the position of the molecular weight markers with a ballpoint pen or pencil. The Ponceau Red stain will be washed off the membrane during the blocking step. Note: Do not let the blot dry out at any step through development, as this will cause an increase in background staining.
Block the blotted membrane in freshly prepared TBS and/or PBS containing nonfat dry milk (3–5% ) (see note on blocking) for 30–60 minutes at room temperature with constant agitation. A maximum blocking time of 2 hours at room temperature should not be exceeded since staining artifacts will appear. If longer blocking times are required, the membrane should be kept at 4°C.
Note on blocking: Soak the blotted membrane in freshly prepared blocking reagent, PBS/3% nonfat dry milk (15gms nonfat milk in 500mLs PBS) for 30 minutes to 2 hours at room temperature with constant agitation. Membranes may also be blocked with PBS/3% nonfat dry milk overnight at 4°C. Generally, phospho-antibodies require blocking reagents diluted in TBS rather than PBS. If previous Western blots had high backgrounds, try a different blocking buffer. Oter blocking reagents which can be used include a) 3–5% Nonfat dry milk/0.05–0.1%Tween, b) Tween-20 (0.05–0.2%) in PBS or TBS and c) 0.2–5% BSA fraction V in PBS or TBS. Generally, maximum blocking time should not exceed 2 hours at room temperature or proteins can be exchanged from the membrane.
Dilute the primary antibody to the recommended concentration/dilution in fresh blocking solution (TBS and/or PBS/3% nonfat dry milk). Incubate the membrane in the primary antibody solution for 1 to 2 hours at room temperature or overnight at 4°C with agitation.
Wash the membrane three times for 3 to 5 minutes each with either water or TBS and/or PBS containing 0.05% Tween 20.
Incubate the membrane in the secondary antibody reagent of choice for 30 minutes to 1 hour at room temperature or overnight at 4°C with agitation. For a mouse monoclonal antibody, a goat anti-mouse HRP-conjugated antibody is recommended, for a rabbit polyclonal antibody, a goat anti-rabbit HRP-conjugated antibody is advisable.
Wash the membrane five times for 3 to 5 minutes each time with either water or TBS and/or PBS containing 0.05% Tween 20.
Note:Tween-20 detergent has the potential to strip low affinity primary antibodies, and therefore the membrane is briefly washed to improve the background. If the membrane has been washed with water, a final wash step for 3 to 5 minutes in TBS and/or PBS containing 0.05% Tween 20 should follow.
Perform the detection of proteins using detection system of choice, (e.g., enhanced chemiluminescence (ECL))
Note: If Tween-20 is not exhaustively removed by washing the membrane with either water, TBS and/or PBS, the Tween-20 could react with ECL reagent, and there may be an increase in overall membrane staining resulting in a black film.
.
Western Blotting
back to top
Detection Substrates
Chromogenic Detection
Prepare the substrate according to manufacturer’s instructions.
Place the blot in a clean container and add substrate to completely cover the surface of the membrane. Incubate for 10 minutes with mild agitation or until signal reaches desired contrast.
Rinse the blot with Milli-Q water to stop the reaction.
Store the blot out of direct light to minimize fading. Blot may be stored dry.
Chemiluminescent Detection
Follow manufacturer’s instructions.
Prepare the substrate according to manufacturer’s instructions.
Place the blot in a container and add substrate to completely cover the membrane. Incubate for 1 minute.
Drain excess substrate.
Note: A cut-to-size sheet protector or a freezer bag can also be used.
Gently smooth out any air bubbles. In a dark room, place the wrapped membrane in a film cassette.
Place a sheet of autoradiography film on top and close the cassette.
Expose film. Multiple exposures of 15 seconds to 30 minutes should be done to determine the optimum exposure time; 1 to 5 minutes is common.
Fluorescent Detection
REQUIRED EQUIPMENT
Proteins blotted onto Immobilon-FL transfer membrane and probed with antibodies
Mylar® wrap
Fluorescent imaging equipment
The following is a general protocol for fluorescent immunodetection. For optimal results, refer to manufacturer’s protocol provided with the reagents.
Note: If using chemifluorescent reagents, follow reagent manufacturer’s directions.
Prepare the substrate according to manufacturer’s instructions.
Place the blot in diluted fluorescent dye-labeled secondary antibody solution and incubate for 1 hour with gentle agitation.
Wash the blot with wash buffer 3–5 times for 5 minutes each.
Place the blot onto a piece of clean filter paper to dry.
If using a wrap, use Mylar. Do not use Saran® wrap because it quenches the fluorescence.
Image the blot using an appropriate fluorescence scanner.
back to top
Rapid Immunodetection Method
Application
Rapid immunodetection takes advantage of the fact that antibodies cannot bind to the hydrophobic (non-wetted) surface of the Immobilon-P transfer membrane, but will bind to a protein immobilized on the membrane. Rapid immunodetection is compatible with both chromogenic and chemiluminescent substrates. The major advantage of rapid immunodetection is that blocking is not required, saving time and eliminating the risks involved (Mansfield, 1994). The method can be used on both dry and wet Immobilon PVDF membranes, enabling the analysis in about 2 hours, as opposed to over 4 hours for the standard method.
Important: When using a dry membrane, the blot must be thoroughly dry before beginning rapid immunodetection (refer to Membrane Drying Methods).
Required Solutions
Primary antibody (specific for protein of interest)
Secondary antibody (specific for primary antibody), labeled with alkaline phosphatase or horseradish peroxidase
Substrate appropriate to the enzyme conjugate
Phosphate buffered saline (PBS): 10 mM sodium phosphate, pH 7.2, 0.9% (w/v) NaCl
Antibody dilution buffer: 1% (w/v) BSA (bovine serum albumin), 0.05% Tween-20 detergent
Methanol, 100%
Milli-Q water
Required Equipment
Shallow trays, large enough to hold blot
Plastic wrap (e.g., Saran), freezer bag, or sheet protector
Autoradiography film and cassette
Dark room and autoradiography film processing equipment
Set Up
When using a dry membrane, dry the blot completely (refer to ‘Membrane Drying Methods’ protocol). Do not re-wet the blot in methanol.
Dilute the primary antibody in dilution buffer to the desired working concentration.
Dilute the secondary antibody in dilution buffer to the desired working concentration.
Note: Enough solution should be prepared to allow for 0.1 mL of antibody solution (primary and secondary) per cm2 of membrane.
Antibody Incubations
Place the blot in the primary antibody solution and incubate with agitation for 1 hour. The solution should move freely across the surface of the membrane.
Place the blot in PBS and wash for 5 minutes.
Place the blot in the secondary antibody solution and incubate with agitation for 30 minutes.
Place the blot in PBS and wash for 5 minutes. Repeat twice with fresh buffer.
Proceed with chromogenic, chemiluminescent or fluorescent protein detection as described under the ‘Standard Immunodetection Method’ protocol.
back to top
High Salt Wash to Remove Persistent Background
Required Solutions
High salt buffer: PBS or TBS buffer supplemented with 0.5 M NaCl and 0.2% SDS
Procedure
Follow the Standard Immunodetection Method through primary and secondary antibody incubations.
After the secondary antibody incubation, place the blot in the high salt buffer and incubate for 30 minutes with gentle shaking.
Rinse blot with Milli-Q water and proceed with chromogenic, chemiluminescent or fluorescent protein detection as described in the Standard Immunodetection Method.
Adding 0.5M NaCl and 0.2% SDS to the wash buffer reduced the background considerably on the Immobilon-PSQ membrane, which as a high protein binding capacity. |
back to top
Membrane Stripping Protocols
Two protocols for removing antibodies from blots are presented below. The first is applicable to any chemiluminescent substrate system and uses a combination of detergent and heat to release the antibodies. The second is commonly used for applications where antibodies have to be separated from an antigen and employs low pH to alter the structure of the antibody in such a way that the binding site is no longer active. Neither method will remove the colored precipitates generated from chromogenic detection systems (e.g., BCIP, 4CN, DAB and TMB). However, it is still possible to analyze the blot with another antibody specific to a different target protein.
Important: The blot should not be allowed to dry between rounds of immunodetection. Any residual antibody molecules will bind permanently to the membrane if it is allowed to dry.
back to top
Re-Blot Plus
Western Blot Recycling Kit
The Re-Blot Plus Western Blot Recycling Kit contains specially formulated solutions that quickly and effectively remove antibodies from Western blots without significantly affecting the immobilized proteins.
Advantages of Re-Blot Plus Western Blot Recycling Kit
No pungent-smelling ß-mercaptoethanol is contained in the Antibody Stripping Solution.
Antibody stripping is done at room temperature. No heating of blots is required.
Blots can be stripped of antibodies in approximately 15 minutes at room temperature.
Blots may be reused in 25 minutes.
Application
The Re-Blot Plus Western Blot Recycling Kit (Cat. No.2500) is effective for removal of antibodies from Western blots that have been developed with chemiluminescence or radioactive iodine or other isotopes.
It is not recommended for stripping colorimetric substrates (TMB, DAB, 4-chloronapthol, etc.), as it is not possible to effectively remove substrates that precipitate at the reaction site. The Re-Blot Plus Western Blot Recycling Kit should be used only for qualitative purposes until it has been established by comparative blot analysis that stripping does not quantitatively affect a given antigen.
Kit Components
Mild Antibody Stripping Solution (10x) – (1 container, 50 mL). (May be purchased separately as Cat. No. 2502.)
Strong Antibody Stripping Solution (10x) – (1 container, 50 mL). (May be purchased separately as Cat. No. 2504.)
Storage
Kit components should be stored at 4°C upon arrival. Product is stable for 3 to 6 months after receipt. If Antibody Stripping Solution crystallizes upon storage, it may be re-dissolved with gentle warming at 37°C before use.
Note: To prevent reagent degradation secure the cap tightly upon storage. Avoid extended exposure to air.
Selection of Reagent
Re-Blot Plus Mild Stripping Solution gives good results on both nitrocellulose and PVDF membranes. However, Re-Blot Plus Strong Stripping Solution will perform better when membranes with high signal are to be stripped or when Re-Blot Plus Mild treatment is not sufficient.
| # of Strips or Blots | Amount of 10X Antibody Stripping Solution | Amount of Distilled Water | Resulting Amount of 1x Working Stripping Solution |
1 Strip | 400 µL | 3.6 mL | 4 mL |
|