CO2恒温摇床解决人胚肾 293 (HEK293) 细胞结团问题(一)
人胚肾 293 (HEK293) 细胞在重组蛋白表达中是最常见的宿主细胞。 这类细胞能够表达大量的膜蛋白,如 G 蛋白偶联受体 (GPCR)
,是无法在最常见的生物制药生产宿主,如:中国仓鼠卵巢 (CHO) 细胞中作表达。 HEK293 虽然是蛋白表达的极好宿主,然而 HEK293
细胞在大规模悬浮培养细胞其结团的问题限制了其在生物过程中的使用。近来许多公司都开发了特殊的培养基配方和抗结团剂,以协助解决这个问题。
本文为一成功案例利用
New Brunswick S41i CO2
恒温摇床进行人类重组蛋白表达的HEK293细胞系从血清贴壁培养驯化至无血清单细胞悬浮培养。驯化过的HEK293细胞系如同典型不结团的悬浮细胞系。我们预期这类细胞系能在生物过程中进行标准的批次或流加培养,如同传统的CHO细胞在搅拌式生物反应器进行扩增培养。
Solving the Aggregation Problem of Human Embryonic Kidney 293 Cells Using the New Brunswick™ S41i CO2 Incubator Shaker
Author
Stacey Willard and Ma Sha
Eppendorf, Inc., Enfeld, CT, USA
Corresponding author: sha.m@eppendorf.com
Abstract
Human
embryonic kidney 293 (HEK293) cells are among the most versatile hosts
for recombinant protein expression. These cells are capable of
expressing large membrane proteins such as G protein-coupled receptors
(GPCRs) that are often not properly expressed by even the most popular
biopharmaceutical production hosts such as Chinese hamster ovary (CHO)
cells. Although an excellent host for protein expression, the issue of
cell clumping in large-scale suspension culture of HEK293 cells has
limited its use in bioprocess. Recently, many commercial entities have
developed specialty media formulations and anticlumping reagents to help
combat this issue. Here, we show a successful example of the adaptation
of a human recombinant protein-expressing HEK293 cell line from
serum-supplemented attachment culture to serum-free single cell
suspension culture using the New Brunswick S41i CO 2 incubator shaker.
This adapted HEK293 cell line behaves like a typical aggregationless
suspension cell line. We expect that such a cell line can be used in
bioprocess applications using the typical batch or fed-batch methods
established for conventional CHO cell culture in stirredtank
bioreactors.
Introduction
Within the
biopharmaceuticals market, the most utilized model systems for protein
production are, by far, mammalian cell lines. Although CHO cells make up
the largest portion of these cell lines, some proteins still require a
human intracellular environment for proper folding, post-translational
modifcation including glycosylation, and function. For this reason,
bioprocess applications involving HEK293 cells have become more relevant
[1, 2]. This versatile human cell line benefts from a long history,
extensive characterization, and successful protein expression in both
transient and stable formats using plasmid and adenoviral vectors. In
fact, in the case of the biopharmaceutical drug Xigris (activated
Drotrecogin alfa), CHO cells were an inadequate host due to improper
glycosylation which rendered the drug unsuitable for human injection.
Used in the treatment of sepsis and marketed by Eli Lilly and Company,
Xigris was the frst biopharmaceutical generated in HEK293 cells to
receive FDA approval [3 – 5].
Recently, a number of studies have
begun to reexamine HEK293 cells as a platform for recombinant protein,
vaccine, and biosimilar manufacturing [1, 2]. One such example involves
the hemophilia treatment, recombinant coagulation factor VIII (rFVIII).
Although classically produced in CHO or baby hamster kidney (BHK) cells,
multiple recent reports have investigated the feasibility of changing
the host cell line to HEK293 [6, 7]. In CHO, the expression levels of
rFVIII are low, leading to higher production costs correlating with
higher biomass requirements. In addition, possibly owing to the improper
protein processing that can occur in non-human host cell lines, the
protein is not efciently secreted. Preliminary studies indicate that
using HEK293 as a host increases manufacturing efciency, reduces.
costs, and eliminates the inclusion of immunogenic post translational modifcations [8].
Several
problems have plagued large-scale HEK293 cell culture. Among them, one
of the biggest hurdles has been that HEK293 cells tend to aggregate in
suspension culture, especially at high cell density. Cell clumping in
suspension culture is extremely detrimental to a growing population
since it restricts the cells inside of the clump from access to sufcient
oxygen and necessary nutrients, leading to increased cell death and
toxin accumulation. To combat this well-known problem, many specialty
media formulations and protocols are now commercially available and a
number of anti-clumping reagents have been developed. Here, we aimed to
evaluate the effectiveness of various anti-clumping methods using a
commercially available human membrane protein-expressing HEK293 cell
line. First, we demonstrate that the adaptation of protein-expressing
HEK293 cells without cell clumping can be accomplished in a single step
in the New Brunswick S41i CO 2 incubator shaker. Second, we show that
the large 35 x 61 cm shaking platform of the New Brunswick S41i allows
for the simultaneous evaluation of a large matrix of cell culture
methods with multiple media formulations and varying doses of
anti-clumping reagents.
Materials and Methods
Attachment cell culture
Untransfected
HEK293 cells used for control experiments were obtained from the
American Type Culture Collection (ATCC®, CRL-1573™). HEK293 cells
expressing hemagglutinin-tagged human Toll-like receptor 4 (hTLR4- HA)
were purchased from InvivoGen® (293/hTLR4-HA). 293/hTLR4-HA cells were
created by stably transfecting HEK293 cells with a pUNO-hTLR4-HA
plasmid. This plasmid encodes the hTLR4 gene fused at the 3’end to the
influenza HA tag. The addition of the HA tag was shown to have no
deleterious effect on the expression and function of the hTLR4 protein by
InvivoGen [9]. Both cell lines, 293 and 293/ hTLR4-HA, were initially
cultivated in Dulbecco’s modifed Eagle medium (DMEM, Life Technologies®,
11960-044) supplemented with 4 mM L-glutamine (Life Technologies,
25030-149), 10 % Heat Inactivated Fetal Bovine Serum (HI-FBS, Life
Technologies, 10438-026) and 1 X penicillin/ streptomycin (Life
Technologies, 15140-122). For the 293/ hTLR4-HA cells, the medium was
also supplemented with 10 µg/mL blasticidin (InvivoGen, ant-bl-1). All
attachment cultures were grown in T-75 flasks and incubated at 37 ˚C with
5 % CO 2 on the static shelf of the New Brunswick S41i CO 2 incubator
shaker. Standard cell culture techniques were used including passaging
using trypsinization with 0.25 % Trypsin-EDTA (Life Technologies,
25200-056).

Figure 1: The New Brunswick S41i CO2 incubator shaker Suspension cell culture methodology
Adaptation
to suspension culture was carried out using multiple methods (Table 1).
Each method is comprised of a commercially-available base medium to
which standard cell culture supplements such as HI-FBS and L-glutamine
were added according to the manufacturer’s adaptation protocol. In order
to maintain plasmid stability and expression, blasticidin was added to
each media formulation at an initial dose of 5 µg/mL. The manufacturer’s
protocol for inoculation and passaging was strictly followed. For
example, the 293 SFM II medium protocol recommended a seeding density of
3 x 105 cells/mL, while the EX-CELL 293 medium recommended 6 x 105
cells/mL. These guidelines were followed in all cases. Cells were
cultivated in single-use 125 mL vented-cap Erlenmeyer flasks (VWR®,
89095-262) with incubator shaker conditions set at 125 rpm, 8 % CO2, and
37 ˚C. The cells were analyzed periodically (at least every 72 hours)
by taking a 1 mL sample and evaluating three parameters: cell density, %
viability, and cell clumping. Viable cell density and viability were
determined using a Vi-CELL® automated cell counter (Beckman Coulter®)
and cell clumping was evaluated by visual inspection under 10 X
magnifcation using an EVOS® FL imaging system (LifeTechnologies).
As
shown in Figure 2, cell clumping was assigned a score from (–)
indicating no clumping to (+++) representing large cell masses, based on
the relative size of the cell clumps. Note that once a method was
successful in producing a suspension culture that satisfed the three
criteria (serum-free, no aggregation, and viable in the presence of
Figure 2: The evaluation of clumping in suspension 293/hTLR4-HA cells:
A: Cells formed large aggregates containing > 50 cells, this culture
received an aggregation score of (+++). B: Successful adaptation in
which the culture showed no evidence of cell clumps greater than 2 – 4
cells; this culture was given a (–) for aggregation. Both images were
taken at the same magnifcation (100 X), scale bars represent 400 µm in
both panels. blasticidin), no more modifcations to the other methods
were attempted. Therefore, full optimization of the media and conditions
was not carried out and we do not know if other formulations would be
successful with further experimentation. In addition, although
blasticidin selection is required for this cell line, it may not be
necessary for other protein-expressing HEK cell lines.
 | 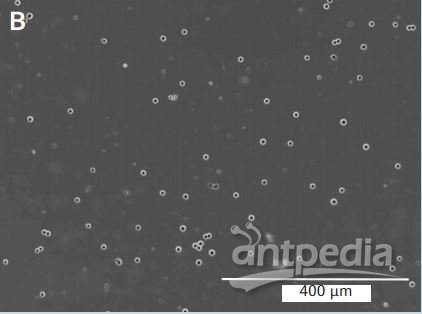 |
Figure 2: The evaluation of clumping in suspension 293/hTLR4-HA cells:
A: Cells formed large aggregates containing > 50 cells, this culture received an aggregation score of (+++).
B:
Successful adaptation in which the culture showed no evidence of cell
clumps greater than 2 – 4 cells; this culture was given a (–) for
aggregation. Both images were taken at the same magnifcation (100 X),
scale bars represent 400 µm in both panels.
Table 1: Culture methods used to adapt adherent 293/hTLR4-HA cells to serum-free single cell suspension culture












