CO2恒温摇床解决人胚肾 293 (HEK293) 细胞结团问题(二)
Lysate preparation and western blotting
Protein
lysates were created by harvesting the cells from confluent T-flasks or
from suspension cultures at high density. Lysates were prepared from
attachment 293 and 293/hTLR4-HA cells as well as from suspension-adapted
293/hTLR4-HA cells grown in EX-CELL 293 medium in the presence of 5
µg/mL blasticidin. The harvested cells were washed with Dulbecco’s
phosphate buffered saline (DPBS, Life Technologies, 14190-144) and
resuspended in the lysis buffer formulation recommended by InvivoGen
(Table 2). Before use, the lysis buffer was sterile-fltered and Halt™
protease inhibitor single-use cocktail (Thermo Fisher Scientifc®, 78430)
was added at a fnal concentration of 1 X. After incubation in lysis
buffer on ice for 20 min, the lysate was cleared by centrifugation at
maximum speed in an Eppendorf Centrifuge 5430 R with a fxed-angle rotor
at 4 ˚C for 20 min. The cleared lysates were stored at -80 ˚C in a New
Brunswick Premium U570 freezer to preserve protein integrity until
western blotting. The protein concentration of each lysate was
determined using the Pierce® BCA protein assay kit (Thermo Fisher
Scientifc, 23227).
SDS-Polyacrylamide gel electrophoresis (SDS-PAGE)
and subsequent immunoblotting were carried out using the following kits
from Life Technologies. First, SDS PAGE was performed using the Bolt™
mini gel system (B4477599) with the accompanying 4 – 12 % Bis-Tris plus
gels (BG04125BOX), MOPS buffering system, and PVDF membranes (B0001 and
LC2002, respectively). 20 µg of each sample was loaded and after
transfer, the membranes were probed with a mouse anti-HA Tag antibody
(InvivoGen, ab-hatag) at a 1:1000 dilution. Detection was performed
using the WesternBreeze® chromogenic western blot immunodetection kit
(Life Technologies, WB7103) with the included anti-mouse secondary
antibody, according to the manufacturer’s instructions.
Immunofluorescence
Attachment
293 and 293/hTLR4-HA cultures were subjected to immunostaining
according to the protocol outlined previously [10]. A mouse Anti-HA Tag
primary antibody was used at a 1:1000 dilution to detect expression of
the hTLR4-HA protein combined with an Alexa Fluor® 594 goat anti-mouse
secondary (Life Technologies, A-11005). Samples were counterstained with
the nuclear dye, 4’, 6-diamidino- 2-phenylindole (DAPI), using ProLong®
gold antifade mountant (Life Technologies, P-36931). Cells were imaged
as described above.
Table 2: Lysis buffer formulation as recommended by InvivoGen

Results and Discussion
Expression of hTLR4-HA in 293 cells
To
confrm that 293/hTLR4-HA cells expressed the tagged hTLR4 receptor,
attachment cells were stained with a mouse antibody raised against the
HA tag and detected with a fluorescent anti-mouse secondary antibody. As
Figure 3 illustrates, varying levels of expression of hTLR4 were
detected in transfected cells, while no signal was found in
untransfected cells. These data indicate that the transfected cells may
represent a pool of transformants instead of a clonal population of
cells. More uniform expression may be obtained if a clonal population
was established.
Adaptation of 293/hTLR4-HA to serum-free single cell suspension culture
To
adapt a human membrane protein-expressing 293 cell line to serum-free
suspension culture without aggregation, 293/hTLR4-HA cells were
subjected to multiple culture methods. Each culture was periodically
analyzed for cell viability, density and clumping as described
previously. As Table 3 indicates, varying levels of success were
documented in each category using the tested media formulations;
however, a successful adaptation was only achieved if the cells retained
high viability and grew to high densities in the presence of
blasticidin with no aggregation under serum-free conditions. If cell
clumping was severe (+++), or viability was low, the culture was
discontinued and adjustments to the method were made accordingly. For
example, DMEM with 0 and 1 % HI-FBS resulted in large cell clumps of
over 50 cells in suspension (Figure 2A). Therefore, after 48 h of
culture, the formulation was adjusted to contain anti-clumping agents
such as Pluronic F-68 and Anti clump A, and a new culture was
established from attachment cells. As outlined in Table 3, DMEM was not
able to support suspension cell growth without aggregation in this
experiment. Another formulation, Pro293 s-CDM, was able to sustain the
growth of 293/ hTLR4-HA with serum supplementation, however, when serum
weaning was complete, the cells did not survive for multiple passages
(Figure 4A). Furthermore, it was clear that many cultures seemed to be
extremely sensitive to blasticidin selection during the adaptation
process as indicated by low viability in the days post-inoculation.
Hence, some cultures were allowed to adapt to suspension culture before
blasticidin was re-introduced. The most successful adaptation method
using this strategy was Method 4 (EX-CELL 293) which, after blasticidin
addition,resulted in virtually no cell clumps and reproducible high cell
densities and viabilities without the presence of serum (Figure 4B).
This method was deemed successful for the adaptation of this cell line
to serum-free suspension culture without aggregation and optimization of
the other methods was halted at this stage. Since no further changes to
the other methods were attempted, it not clear whether or not other
methods would have been found successful in future experiments.
 |  |  |
Figure 3: hTLR4-HA
expression in untransfected (A) and transfected (B, C) 293 cells. In all
panels, hTLR4-HA is detected in red and DAPI in blue.
A and B: Photographed at 200 X magnifcation, scale bar = 200 µm.
Panel C: Photographed at 400 X, scale bar = 50 µm.
Table 3: Result of suspension cell culture with the methods and formulations tested

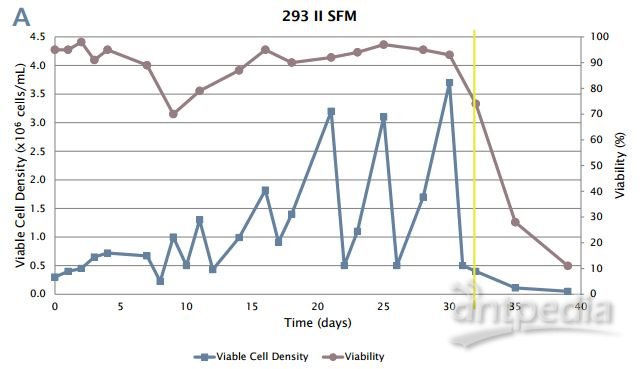

Figure 4: 293/hTLR4-HA cell adaptation to suspension culture.
A:
Cells were able to adapt to suspension culture without serum, but died
upon addition of blasticidin (yellow line). When blasticidin was present
at the beginning of adaptation in this method, the cells died rapidly
(data not shown).
B: Successful adaptation to single cell serum-free
suspension culture. The yellow line denotes the addition of blasticidin.
Note that after 45 days, the cells begin to grow reproducibly over
multiple passages at high viability.
Protein expression after suspension adaptation
When
acclimating a cell line to suspension culture in preparation for
scale-up to a stirred-tank bioreactor, it is important to confrm that
protein expression was not impacted by the adaptation process. Whole
cell lysates from untransfected 293 and 293/hTLR4-HA were analyzed for
hTLR4-HA expression by western blotting. As shown in Figure 5, no signal
was detected in whole cell lysates from untransfected (293) early or
late passage adherent cells. In contrast, early passage adherent
293/hTLR4-HA cells expressed a ~97 kDa HA-tagged protein.
Suspensionadapted cells cultured in EX-CELL 293 media with 5 µg/mL
blasticidin expressed an identical band of approximately the same
intensity. This band closely matches the predicted size of the human
TLR4 protein at 95 kDa with ~1 kDa added for the 9 amino acid HA tag.
Although not quantitative, these data indicate that the expression of
hTLR4-HA was not signifcantly impacted by the adaptation process.

Figure 5: Post-adaptation hTLR4 expression confrmation by western blot.
Untransfected (293) attachment cells of early (E; passage 3) and late
(L; passage 21) passage and 293/ hTLR4-HA attachment (A) and
postadaptation suspension (S) cell lysates were probed with anti-HA
antibody and detected by the WesternBreeze chromogenic detection method.
20 µg of protein was loaded in each lane.
Conclusion
The
number of FDA-approved biopharmaceuticals produced in HEK293 cells has
been low, partially due to the wellknown large-scale suspension culture
aggregation issue in bioreactor conditions. In this work, we eliminated
the clumping problem in our HEK293 cell line prior to the bioreactor
production stage, leveraging commercially available serum-free
adaptation methods. We have shown that the adjustment of a membrane
protein-expressing HEK293 cell line to clump-free serum-free suspension
culture can be accomplished by simultaneously testing multiple
adaptation methods in the New Brunswick S41i CO 2 incubator shaker. This
method cuts down on upstream process development time since it can
support adherent and suspension cells simultaneously in the same
chamber. Moreover, the large shaking platform can accommodate up to
twenty-four 125 mL flasks. By eliminating aggregation before the
bioreactor stage, we hope to address one of the major bottlenecks that
has limited the bioprocess potential of this cell line.
Literature
[1]
Liste-Calleja L, Lecina M, Cairo JJ. HEK293 cell culture media study
towards bioprocess optimization: Animal derived component free and
animal derived component containing platforms. Journal of Bioscience and
Bioengineering 2014; 117(4):471-7.
[2] Dietmair S, Hodson MP, Quek
LE, Timmins NE, Gray P, Nielsen LK. A multi-omics analysis of
recombinant protein production in Hek293 cells. PloS One 2012;
7(8):e43394.
[3] Doig CJ, Zygun DA, Delaney A, Manns BJ. Drotrecogin
alfa (activated; Xigris): An effective and cost-efcient treatment for
severe sepsis. Expert Review of Pharmacoeconomics & Outcomes
Research 2004; 4(1):15 – 26.
[4] Levi M, De Jonge E, van der Poll T.
Recombinant human activated protein C (Xigris). International Journal of
Clinical Practice 2002; 56(7):542 – 5.
[5] Larson AM. Xigris: Reducing mortality in adult patients with severe sepsis. Urologic Nursing 2002; 22(3):200-1.
[6]
Fontes AM, Melo FU, Greene LJ, Faca VM, Lin Y, Gerson SL, Covas DT.
Production of human factor VIII-FL in 293T cells using the bicistronic
MGMT(P140K)-retroviral vector. Genetics and Molecular Research : GMR
2012; 11(1):775 – 89.
[7] Swiech K, Kamen A, Ansorge S, Durocher Y,
Picanco-Castro V, Russo-Carbolante EM, Neto MS, Covas DT. Transient
transfection of serum-free suspension HEK 293 cell culture for efcient
production of human rFVIII. BMC Biotechnology 2011; 11:114.
[8]
Valentino LA, Negrier C, Kohla G, Tiede A, Liesner R, Hart D, Knaub S.
The frst recombinant FVIII produced in human cells--An update on its
clinical development programme. Haemophilia 2014; 20 Suppl 1:1 – 9.
[9]
HEK-293 cells stably transfected with human HA-tagged TLR4a gene.
InvivoGen, Inc. 2014 Version # 14G30-MT www. invivogen.com.
[10] Willard S, Philip L, Sha M. Hypoxic Cell Culture in the New Brunswick™ Galaxy® 170R Incubator: Normal Growth, Morphological Changes. 2013. http://www.nbsc.com/fles/AA331_Hypoxia_CO2_Incubators.pdf





















