The effect of lithium chloride on Lytechinus variegates embryos
Purpose
This experiment will study effects of lithium chloride on sea urchin development, forcusing on archenteron formation.
Introduction
Gastrulation is extensive cell rearrangement where cells undergo dramatic movements and change relative positions. From this ordered movement, layers of cell are created. The cells that will form the endodermal and mesodermal layers, and then organs, are brought inside the embryo, while the cells that will form the skin and nervous system spread over the outside surface. The three germ layers - outer ectoderm, inner ectoderm, and interstitial mesoderm - are produced during gastrulation (Gilbert, 1997).
After the sixth cleavage in normal sea urchin, two tiers of eight cells each are formed in the vegetal half of the embryo. The top tier is termed veg1 and the bottom tier is termed veg2. Veg1 lineages have been shown to contribute portions of the definite hindgut, midgut, and the ectoderm that surrounds the blastopore at the completion of gastrulation (Cameron, 1997). Veg2 and micromere lineages normally contribute to secondary mesenchyme, the coelomic sacs, as well as most of the archenteron (Cameron, 1997). High levels of B-catenin in micromeres prior to gastrulation suggest that B-catenin plays a signaling role (Miller and McClay, 1997).
introduction cont. Embryos that have been treated with lithium chloride accumulate B-catenin in every cell (Gilbert, 1997). LiCl inhibits GSK-3, a B-catenin regulating molecule, leading to higher B-catenin levels (Logan et al., 1999). Developmental abnormalities in gut formation accompany increased nuclear B-catenin levels achieved with LiCl treatment. This study examined the effects of LiCl on sea urchin gastrulation by examining embryo development in different concentrations of a LiCl solution. It is expected that allowing sea urchin embryos to develop in LiCl will disturb normal vegetal cell processes due to excess B-catenin (Cameron and Davidson, 1997).
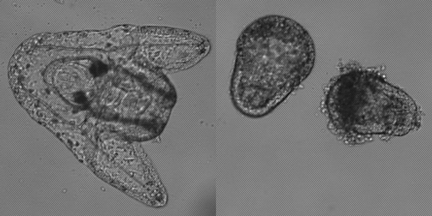
Lithium has been proven to cause abnormally exaggerated development of structures derived from the vegetal area. Lithium chloride can also result in the formation of embryos with a proportionally large archenteron or even with an archenteron that bulges outward from the surface rather than invaginating properly into the blastocoel. This phenonmenon shown above is known as exogastrulation. Procedure I. Animals: Strongylocentrotus purpuratus, from the Pacific coast, Arbacia punctulata, from the Atlantic Coast or Lytechinus variegatus, from Florida can be used for this experiment. S. purpuratus gametes and embryos must be kept close to 12- 13ºC, or they will not develop. Gamete collection by induced spawning 1. Induce spawing of gametes and fertilize as described in the basic protocols. 2. Collect both sperm and eggs. II. Treatment with LiCl Solutions and Examination: 1. Use a 60 mM solution of LiCl in ASW to prepare 30 mM and 15 mM solutions. 2. At the two-cell stage, transfer embryos from ASW to seawater with each of the three LiCl concentrations. A quarter of the embryos should remain in ASW as a control group. 3. At the mesenchyme blastula stage, transfer 50 ml of embryos in finger bowl to ASW. 4. Observe samples of embryos from a control (ASW with no lithium added) culture and each of the experimental cultures the next day. 5. Use 2-3 drops eggs/embryos on a depression slide to compare developmental rate and morphology of the groups; focus on vegetal area morphology of embryos. III. Immunofluorescent staining of archenteron Preparation of fixed embryos 1. Centrifuge 50 ml embryo cultures for 5 minutes at 1500 rpm. Check for pellet of embryos at the bottom. Quickly decant as much ASW as possible. 2. Gently swirl tube to resuspend the embryos. Add 40 ml of ice cold methanol and allow to fix on ice. Embryos should have settled to the bottom of the tube. 3. Decant methanol and resuspend the embryos in 25 ml ice cold ASW. 4. Let the embryos settle to the bottom of the tube by gravity on ice. 5. Decant ASW and resuspend the embryos in fresh ice cold ASW. At this point embryos can be stored in refrigerator. 6. Let the embryos settle on ice again. Decant most of ASW, leaving 5-10 ml. Swirl to resuspend in remaining ASW. Transfer to 1.5 ml microfuge tubes. 1. Allow 2 tubes fixed embryos to settle for 10 minutes. Gently decant liquid. 2. Add 200 ul of 5C7 antibody to one tube and 200 ul 10% normal goat serum to the other. Incubate 45 minutes at room temperature. Embryos will settle. 3. Decant as much liquid as possible. Add 1 ml ASW to wash. Allow to settle, decant. 4. Add 200 ul Texas Red-congugated Goat anti-mouse IgG to both tubes. Incubate 45 minutes at room temperature. Embryos will settle. 5. Decant as much liquid as possible. Add 1 ml ASW to wash. Allow to settle, decant. Add 100 ul PBS. 6. Transfer 10 ul of each sample to slides. Check that there are embryos. Place coverslip on slide. Examine using epiflourescence. Solutions 60 mM LiCl in sea water |