Differential Interference Contrast
Differential Interference Contrast (Nomarski, DIC, Hoffman Modulation Contrast)
Principle
Differential interference microscopy requires several optical components, therefore it can be very expensive to set up. Light from an incandescent source is passed through a polarizer, so that all of the light getting through must vibrate in a single plane. The beam is then passed through a prism that separates it into components that are separated by a very small distance - equal to the resolution of the objective lens. The beams pass through the condenser, then the specimen
In any part of the specimen in which adjacent regions differ in refractive index the two beams are delayed or refracted differently. When they are recombined by a second prism in the objective lens there are differences in brightness corresponding to differences in refractive index or thickness in the specimen. Regions such as the edge of a cell or nucleus are very distinct because the quality of the specimen changes so much over a very short distance.
One or more components of the system are adjustable to obtain the maximum contrast. When the contrast is optimized one can obtain a very distinct image that appears three dimensional. The effect is very much like what you see when a subject is shadowed by a strong light coming from one side, as with craters on the moon near the terminator, namely the boundary between the sunlit portion of the Moon's surface and the dark side.
Mimicking a DIC effect
If you have a condenser that is adjustable, that is, it can be placed off center, you can produce a shadowing effect that is very much like a true DIC image. You simply need to experiment to find the best position for the condenser for each objective lens. Generally you need a lot of light, since the effect is obtained by selective reduction of illumination.
Exercise: observing pseudopodia of Chaos carolinensis
Prepare a vaseline mount of Chaos carolinensis (also called Pelomyxa Carolinensis). Chaos is a large amoeboid protist that is very common in detritus in ponds and in the solid portion of sewage. The heavily granulated cytoplasm makes a very nice model for observing cytoplasmic streaming.
Find the specimen using dark field mode at 40x. Chaos are very large, so this shouldn't be much trouble. Move to 100x, focus, then go to bright field. Now move the condenser slightly away from the bright field position until the image appears three-dimensional. This works with condensers that have turrets that rotate for different modes of viewing.
Most amoebae are negatively phototropic, that is, they shy away from light. As the amoeba moves it will shoot out pseudopodia that are well attached to a glass surface (the surface could be the slide or the underside of the cover slip). Pseudopodia are very thin, so you can see a lot of detail. The detail shows up dramatically with a differential interference contrast effect.
Applied Microscopy
Here are a few exercises with the microscope that are designed to help you practice and retain your new skills. For each study an initial procedure is recommended. After that, please try different modes yourself. The optimum mode for viewing varies with the specimen, how it behaves or is prepared, and your own objectives.
Preparing a wet mount (Vaseline mount)
Living specimens often do not survive long in the heat from an intense microscope illuminator bulb, usually because the specimen dries up. This problem is easily solved by preparing a vaseline chamber. Simply take a single cover slip and hold it between thumb and forefinger by the edges. Pick up some vaseline on the other forefinger and rub it over your thumb to make a film. Scrape your thumb carefully on each edge of the coverslip to make a continuous vaseline ledge.
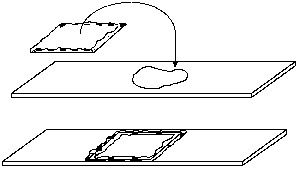
Place a drop or two of suspension on a clean slide, and turn the coverslip over on top of the drop. Press down the edges to seal the chamber against evaporation. When preparing a vaseline mount, keep in mind that the image becomes degraded with thicker mounts, especially at high powers in dark field or phase contrast. Unless the specimen is large and fragile enough to be damaged by pressing down too hard on the coverslip, keep the chamber depth very shallow.
Digestion of yeast by Paramecium
Paramecium feed by swimming through regions that are rich with smaller protists, yeast, large bacteria, and other readily-ingested digestible materials. They move and feed by beating their cilia. Food organisms are brought into a crevice called the buccal cavity, at the end of which endocytosis takes place forming a food vacuole.
Obtain a drop of concentrated paramecium and add a dab of heat-killed yeast stained with Congo red dye. The yeast suspension consists of 30% compressed yeast, 0.3% Congo red in distilled water. It is boiled gently for 10 min. then cooled to room temperature before use. Prepare a vaseline mount of the yeast-Paramecium mix and begin observing in dark field, starting with the lowest power.
Paramecium move rather quickly, thus that they can be difficult to chase and observe. They may slow down to feed, however, and may congregate after awhile especially if there are clumps of yeast that keep them busy. Observe the manner in which they move, the beating action of cilia, specific organelles, and the digestion process. As the yeast are digested the pH changes inside the food vacuoles. Congo red dye is pH-sensitive, so as digestion proceeds the color changes from bright orange-red to purple to blue. Dark field distorts the colors, but you may find the effect to be more dramatic.
The cilia themselves can be seen if you get the paramecium to quiet down. If they won't settle right away, set the slide aside and try again later. Cilia show up best in phase contrast (400x), or in dark field at 100x if you have sharp eyes. You might also get to see a contractile vacuole as it fills and ejects water, the nucleus, and perhaps one or more micronuclei.
Ingestion of Paramecium by Chaos
Prepare another vaseline mount of Chaos, only this time add a drop of concentrated paramecium as well. Begin observing immediately. The food chain in freshwater communities starts with bacteria, which are eating by a host of organisms including small protists. Paramecium, among other protists and metazoans, feed on the protists, and lots of organisms feed on paramecium. I think of paramecium as the "cattle" of freshwater laboratory cultures. They are good for maintaining larger predatory organisms, including Chaos.
Start with low power, as usual, observing the Chaos. You'll be seeing the paramecium soon enough. You may be wondering how something as sluggish as an amoeba can catch and consume something as fast as a paramecium. Your observations should give you a clue. Once the Chaos has captured one or more paramecium you may wish to use bright field mode and stop down the aperture diaphragm to enhance contrast within the food vacuole (or try getting a DIC effect).
Fixation of Chlamydomonas and flagellar staining
Obtain 8 or 10 drops of a sample of living, motile Chlamydomonas in a culture tube. Prepare a vaseline mount of the culture and observe the living organisms. You'll need to use dark field at a low power (100x) to locate them, as they are very small and very fast. Zoom in on one or more cells that aren't moving, using 400x in phase contrast mode. Can you see one or more flagella?
Add a drop or two of Lugol's iodine to what remains in the culture tube (ratio should be 4:1 culture to iodine). Mix and prepare a slide of one drop of culture, with cover slip but no vaseline. The cells won't be moving now, so they will be harder to see. They appear yellow to reddish-brown in dark field mode. See if you can spot the flagella now, which will appear bright against the dark background. Zoom in on one or more cells as before, and look for flagella. They appear dark in phase contrast. Try to measure them using the ocular micrometer scale.
Observations of living bacteria
Obtain a bacterial plate or broth culture. Have handy a Fisher burner, striker, squirt bottle and loop. Turn on the gas, light the burner, and flame the loop as shown. Dip the loop in the broth culture, using aseptic technique as shown. Transfer the loopful of material to a slide and drop on a cover slip. Alternatively, place a small drop of water on a clean slide using the squirt bottle. After flaming the loop cool it by touching the surface of a bacterial plate, then, using aseptic technique, pick up a small amount of material and mix it into the drop of water. Drop on a cover slip.
Use dark field at low power to find the suspension, focusing first on a bubble or cover slip. the bacteria will be visible even at the lowest power, as bright spots against a dark background. Work up to 400 power. When you go from 100x to 400x, go to phase contrast mode, then swing the 40x phase contrast lens in place and focus. You may need to adjust the phase centering screws to get good contrast. Living non-motile bacteria exhibit Brownian motion, that is, they vibrate due to molecular motion. Motile bacteria will rapidly cross the field of view or lumber along in various directions depending on their size.
Mixed freshwater cultures (collected specimens)
An incredible variety of living things can be observed in samples taken from ponds, streams, stagnant pools, and cultures maintained in the laboratory. Collected samples and laboratory cultures can support a number of investigations that are centered on microscopy as the primary research tool.
Laboratory cultures as well as collected samples should be available for observation. First, place specimens in the wells of a micro spot plate and observe in the dissecting microscope (start at low magnification). Adjust the light to get a dark field effect, so you don't miss anything. The plate should be elevated on an illuminator stand or equivalent and the lamp aimed at the specimen either horizontally through the plate or at a 45 degree angle above the plate onto the specimen. Observe the variety of living things and how they move, attach, feed, etc.
Select a specimen for viewing at higher magnification. Remember that many organisms attach to surfaces or congregate around particles, so include some living plant material or detritus in your specimen. Make a wet mount and begin observing.
As your skills improve, you can find more and more living things in a seemingly sterile environment. We will try to provide cultures with a diverse assortment of organisms, however their availability depends on the weather and degree of pollution of freshwater sources. The greatest diversity is in relatively stable, established, unpolluted ponds and slow streams. Drainage ditches such as Houston's bayous clear out after heavy rains, so that many times the samples are disappointing. They are also rather polluted, so that organisms that tolerate murky waters and lack of oxygen thrive, while more sensitive organisms are nowhere to be found.
You may wish to bring in your own samples. Any material from an untreated duck pond or backyard lily pond (preferably in full sun), a bog or marsh, a very green, stinky tank (pond), or a stagnant pool from a sunny location, associated with a stream, can be host to a diverse population of "critters."







