Microscopes in Cell Biology
Microscopes in Cell Biology
Introduction
Microscopy has a major role in the study of cells. From the very beginning, researchers have tried to develop ways of looking directly at living cells. This direct examination has revealed much about the morphology of cells and tissues. In recent years, developments in microscopes, dyes, staining protocols and preparation techniques have helped reveal even more about the structure and function of cells. The department of Advanced Electronmicroscopy and Imaging has many microscopes available
Continued, efficient use of these microscopes in modern cell biology and the production of good quality micrographs comes from a good knowledge of these instruments. The researcher, although challenged by rapid technical advances, must understand the basic principles of microscope operation and their technical limitations.
The aim of this section is to introduce, in simple terms, the capabilities and limitations of the optical components of the light microscope, the adjustments required for optimal use of the instruments, specimen preparation procedures and photographic techniques.
The Light Microscope in Biology
Photomicroscopy
The technique of making photographs by means of an optical microscope is called photomicroscopy. In its simplest form an ordinary 35mm camera can be attached to the ocular tube of a microscope and the image recorded on photographic film. The quality of the image formed will depend almost entirely on the quality of the image produced by the light microscope. This in turn is dependent upon the quality of the lenses used for the imaging and upon the correct adjustment of the microscope.
The optical system of a light microscope can be separated into three parts. These are the substage condenser lens, the objective lens and the eyepieces.
Light, from the illumination source, is directed through the substage condenser lens (found below the specimen stage) which focuses it onto the specimen (mounted on a glass slide). This light then passes through the specimen and into the objective lens. It is this lens that resolves the fine detail present in the specimen. It projects the magnified image to a fixed position behind the lens, in the body of the microscope where it is further enlarged by the eyepieces (or oculars). The light emerging from the oculars is focussed to a point, called the eyepoint, where the eye can see the magnified image.
The condenser lens
On most microscopes, light entering the condenser lens usually comes from a fixed light source at the bottom of the microscope. The size of the light beam entering the lens is controlled by the field diaphragm in front of the illumination source. Adjustments of the focus of the condenser lens and the opening of the diaphragm are performed for Kohler illumination (see below).
The objective lens
Microscope objectives come in many forms, which are all interchangeable on the microscope. A light microscope usually has a selection of objective lenses on the nosepiece. The most obvious difference between these objectives is the magnification power of each. However, objective lenses can also differ in their degree of optical correction, their numerical aperture and their tube length.
The eyepieces
The purpose of the eyepieces in a light microscope is to further enlarge the primary image formed by the objective lens and to render it visible as a "virtual" image for the eye to see. Although the eyepieces do not improve the resolution of the image they magnify, low quality eyepieces can degrade the image formed.
Other parts of the system
Although the quality of the lenses used in the construction of a light microscope play a major role in image quality and resolution, they are also affected by other external factors.
The specimen
One important fact that is often overlooked in microscopy and photomicroscopy is that the specimen is a part of the optical system. It is important that sections through tissues or cells be as thin as possible to obtain maximum resolution. Although it is possible to obtain sections of frozen or plastic embedded tissue as thin as 0.3 to 2 µm, most researchers still use 10 to 20 µm thick sections for their work. Examination of whole cell mounts results in an unavoidable loss of resolution.
The coverslip glass
Although the majority of the coverslips commercially available today are made from optical quality glass and are in the correct thickness range of 0.16 to 0.19 mm, the quality and thickness of the coverslip glass affects the microscopic image. Coverslip thickness is less critical if oil immersion lenses are used when the refractive index of the glass is the same, or close, to that of the immersion oil. Image quality, however, can be affected if the coverslips are contaminated with mounting medium, immersion oil or dirt.
Vibration
The image produced by all cameras is affected by vibration. The long exposure times sometimes used in light microscopes makes these instruments very susceptible to vibration. This may be external vibration, coming through the base of the microscope, or it may originate from the microscope itself. Most microscopes are designed to have low levels of vibration during normal operation so if the apparatus is placed on a solid support away from obvious sources of vibration (e.g., elevator shafts), then problems with vibration should not occur. Touching the microscope or the support table when picture taking, however, may cause vibrations which will affect the final image.
Calculating the final magnification on the photomicrograph
The magnification of the image on the film is the product of the objective magnification and the eyepiece magnification. For microscopes with cameras separated from the eyepieces, the magnification of the image onto the film is substituted for the eyepiece magnification.
A more accurate and simpler method is to measure the magnification using a stage micrometer. This is a glass slide onto which is etched a scale of finely ruled lines of known separation (usually in millimeters). The stage micrometer is placed on the specimen stage and its image recorded on film. Measuring the separation of lines and comparing these with the original scale will give an accurate estimation of the magnification of the image. This estimation can be performed on the film, or on the final image to be used.
A convenient formula for calculating the magnification is
magnification = Image size/object size
Use of immersion oil
High magnification and high resolution are obtained in the light microscope by using oil immersion objective lenses. When an objective lens is to be used in this way it will have the word "oil" written on it. For general use, a drop of oil is placed on the coverslip of the specimen slide and the objective lens is immersed into the oil. Only small amounts of oil are needed, and the objective lens should be cleaned of oil after use by wiping it with a piece of lens tissue. Take care not to contaminate the microscope with the oil, especially any objective lenses that are not oil immersible.
Objective lenses with a numerical aperture [NA] of more than 1.00 will work better if the condenser lens is also an oil immersion lens. This type of condenser lens is not available on all microscopes.
When using the oil, it is important not to shake the bottle or introduce air bubbles by any other way. The bottle should never be left open for long periods of time, as dust and other debris in the oil will impair the image quality. Contamination of the immersion oil with the specimen mounting medium (which will interfere with the final imaging) can be avoided by either letting the medium dry before examining the slide or by sealing the coverslip to the slide with nail polish. In addition to affecting the image resolution, contaminated immersion oil will produce high levels of background fluorescence when illuminated with UV light.
Protocol for using oil immersion lenses
Find the specimen using a low power dry objective lens.
Move the oil immersion lens into position and place a small drop of oil on the coverslip. To prevent air bubble formation, a drop of oil can be placed on the objective lens.
Move the specimen stage up to the objective until the lens is near, but not, touching the slide.
Look through the eyepieces and slowly move the specimen slide away from the objective lens until the specimen on the slide is in focus.
This simple procedure can be remembered as "rack up, focus down."
Methods of illumination
Kohler illumination
This is the most widely used system of illuminating a specimen in photomicroscopy. It is the illuminating system that provides best image quality and illumination.
Kohler illumination uses a field condenser to focus the image of the lamp onto the substage condenser, which in turn focuses the image of the lamp condenser onto the specimen.
The main advantage of this system of illumination is that, when the elements are properly aligned, a uniformly illuminated field is projected onto the specimen. Using this system, there is no restriction on the light source, so different types of bulbs can be used.
Protocol for alignment of Kohler illumination system
Turn on the light source and open the field diaphragm.
Place a slide on the specimen stage and focus the specimen with the objective lens.
Close down the field diaphragm to a small aperture.
Focus the field diaphragm image by moving the condenser up or down.
Center the field diaphragm image.
Open up the aperture of the field diaphragm until its diameter is equal to or just less than the observed field in the microscope.
Darkfield illumination
Although not in general use for cell biology, it is worth mentioning this method of illumination. For darkfield illumination, the cone of light illuminating the specimen must not enter the microscope objective. Only light that is scattered by the specimen is detected by the objective lens. This is achieved by use of darkfield diaphragm stops or special darkfield substage condensers called paraboloid or cardioid condensers.
The essential principle of darkfield optics is the formation of a hollow cone of light whose apex falls on the specimen plane. If the light is focused at the object plane and there is no object present, then the objective lens is inside the dark base of the hollow cone of light and the light is invisible. When a specimen is present, the light is deviated, or scattered, by structures on the specimen into the objective lens. A bright image of these details is then visible against a dark background. Because of the high image contrast produced, this illumination method is able to detect extremely fine particles. No artificial specimen contrast is needed to detect fine detail, so this method is useful for examining living cells. It cannot, however, visualize intracellular structures.
Specialized imaging techniques
Phase contrast
Phase contrast microscopy is probably the most widely used method of examining living cells. It can be used to produce contrast effects of many low contrast specimens, such as living cells, and can visualize intracellular structures.
Theoretic aspects
When a ray of light from a single point source is split in two and passed (refracted) through a transparent medium, the two rays can be recombined without interference. If one of the rays, however, passes through a medium of a different refractive index, its speed is altered and the two rays, when recombined, may be out of phase. If this happens then interference occurs and the recombined beam is less bright than the original beam.
A way to visualize this interference is to imagine the split rays as waves. If neither one is altered then they can recombine in phase, with no loss of intensity. If one of the rays, however, has its speed changed, the waves may no longer match when recombined. When the configurations differ by less than a wavelength, the waves are out of phase and their recombination results in a loss of intensity. When they are out of phase by 1/2 wavelength, the rays will cancel each other out, and no light is seen.
Practical aspects
In the light microscope, phase contrast effects are obtained by the use of a ring-shaped annular slot located near the condenser lens, which becomes the light source for illuminating the specimen. A mask is then positioned behind the objective lens in the aperture plane, where the annular light source comes to focus. The mask in the objective is called a 1/4-wave ring because it will advance or retard the light by 1/4 of a wavelength. This is done because it has been found that the best rendition of detail usually occurs when most of the details in the two beams are out of phase by 1/4 of a wavelength. The quality of the image produced will depend on whether the light has been advanced or retarded by 1/4 of a wavelength. In one case a positive image is produced and in the other, a negative image. The image appearance is indicative of the type of contrast produced. If the specimen is brighter than the background then bright phase contrast is being used; when darker, dark phase contrast exists.
In addition to being affected by the interference of rays of light, contrast can also be affected by the medium in which the specimen is mounted. If the refractive index of the medium is too close to that of the specimen then the contrast will be reduced. A good medium to use is Mowiol.
Contrast can be further enhanced by using a green filter (not recommended for color films!) over the light source. Wratten filters No. 58 or No. 61 are recommended by Kodak.
Interference contrast
Another widely used method of enhancing contrast in low contrast specimens is called interference contrast (or differential interference contrast - DIC). This method does not yield the level of contrast enhancement obtained by phase contrast, but it does eliminate many of the peripheral and structural edge halo effects produced by phase contrast. As a consequence, interference contrast will delineate fine detail within specimens. It also produces a color effect and gives a three-dimensional relief to the specimen.
Comparison between phase contrast and interference contrast optical systems
Apart from the fact that the condenser is fully illuminated, the basic difference in the optics of the two systems is that the specimen produces the two interfering beams in phase contrast, but it is the optical system which produces them in the interference contrast method. The interference contrast beams are called the object beam and the reference beam. The first carries the image, and the second is either a homogeneous or an asymmetrical beam. Interference takes place between the two types of beams, not between two image components as in the phase contrast system.
Once the object and reference beams are split, they are separate in the plane of the specimen, but they are recombined for interference before they reach the eyepieces. There are two commonly used methods for separating the beams. These are the double focus system and the shear system. In the first, both beams are focused by the condenser along the optical axis of the microscope. The object beam is focused in the plane of the specimen and the reference beam is focused above or below the slide and forms no structured image. In the shear system, both beams are focused onto the specimen but they are optically displaced laterally. In effect, the specimen is only illuminated by the object beam. The reference beam is moved to one side for subsequent recombination below the eyepiece. As the reference beam should pass through a relatively clear area of the specimen to minimize imaging of unwanted detail, this system is more suitable for examining specimens which are not tightly packed.
Nomarski differential interference contrast
A commonly used interference system was worked out by Nomarski and employs a modified shear system.
The light passes through:
A polarizing filter to produce a beam of polarized light.
A Wollaston prism to split the beam in two and rotate the planes of polarization of the two beam so that they are perpendicular to each other.
The condenser, the specimen and the objective.
A second Wollaston prism to recombine the beams.
A second polarizing filter to rotate the planes of polarization so that the beams can interfere with each other before entering the eyepieces to form the interference contrast image.
Fluorescence microscopy
When excited by radiation of short wavelengths, some substances will emit light of a longer wavelength. This phenomenon is called fluorescence. The usual light source for excitation radiation is ultraviolet (UV) light, but blue light can produce the same effect. Some substances possess autofluorescence, but others which do not normally fluoresce can be impregnated with dyes which will fluoresce. These dyes, or fluorochromes, can also be attached to proteins such as antibodies, which can then be used to detect specific proteins within cells and tissues sections by light microscopy.
For the detection of fluorescent substances in the light by microscope, the specimen is usually illuminated by a beam of filtered (UV) light passed through the objective lens. The light source is a high-pressure mercury vapor lamp which emits very bright radiation in both the UV and short-blue wavelengths. The filter will freely transmit UV light on its way to the specimen but will absorb all visible light which is not required to produce fluorescence. After the fluorescence has been produced, the visible light passes into the objective lens and through a barrier filter which will only allow passage of light produced by the specimen.
The fluorescent images produced can be recorded on either black-and-white or color film. Long exposure times and high speed film are usually required to obtain photomicrographs of fluorescent specimens. Daylight-type color film works best because of its balanced sensitivity to red, green and blue.
-
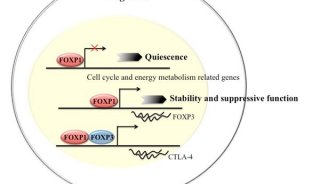
科技前沿

-
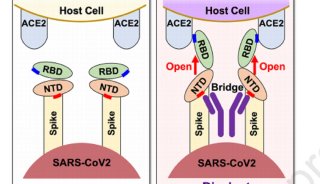
科技前沿

-
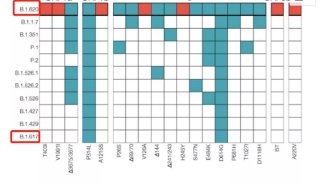
项目成果
-
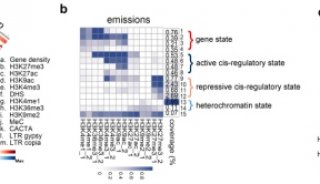
科技前沿
-
科技前沿