蛋白质定量
Quantitative Determination of Peptides by Sulfhydryl (-SH) Groups New (Contributed by David Van Horn, Dept. of Chemistry, UC Berkeley Greg Bulaj, Dept. of Biology, University of Utah)
This is the standard “Ellman’s Test” for the determination of free thiols. It works well for small peptides and proteins synthesized using standard solid phase synthetic methods.
Lowry Protein Assay (P.J. Hansen Lab, Dept. of Animal Sciences, University of Florida)
The Lowry procedure is one of the most venerable and widely-used protein assays, being first described in 1951 [Lowry et al., J. Biol. Chem. 193: 265-275 (1951)]. Under alkaline conditions, copper complexes with protein. When folin phenol reagent (phospho-molybdic-phosphotungstic reagent) is added, the Folin-phenol reagent binds to the protein. Bound reagent is slowly reduced and changes color from yellow to blue.
Lowry Assay for Protein Concentration (Folin-Ciocalteu Assay)
Lowry Protein Assay (William H. Heidcamp)
Bradford Protein Assay (P.J. Hansen Lab, Dept. of Animal Sciences, University of Florida)
The Bradford protein assay is a simple procedure for determination of protein concentrations in solutions that depends upon the change in absorbance in Coomassie Blue G-250 upon binding of protein. Unlike many other assays, including the Lowry procedure, the Bradford assay is not susceptible to interference by a wide usr/localiety of chemicals present in samples. The notable exception is high concentrations of detergents. There is significant protein-to-protein usr/localiation in absorbance values obtained with the Bradford procedure and it is advisable to choose a protein standard that is likely to give absorbance values close to those for the protein samples of interest. The assay here is designed for use in microtiter plates. This is an easy assay format for those with access to multiple channel pipettors and microtiter plate spectrophotometers.
Bradford Protein Assay (William H. Heidcamp)
Biorad Protein Assay: Bradford Reagent (Suprya Jayadev)
Biuret Protein Assay (William H. Heidcamp)
Modified Lowry Protein Assay (Hancock Lab)
The advantage of this assay over the Lowry assay from which it is derived, is the presence of SDS in reagent A. This allows one to perform protein assays in nearly any detergent (eg., when detergents are used in the purification of outer membrane proteins). In addition, SDS separates membrane proteins from contaminating membrane constituents and denatures the proteins allowing more reproducible results.
Micro-Protein Assay (Jim Brown Lab)
This micro-protein assay is not as accurate as other methods for assaying protein concentration, but it has the distinct advantage of requiring only trace amounts (less than 1ug) of your valuable protein. In many cases the accuracy (within a factor of 2) of the assay is more than sufficient. Another advantage of the assay is its relative ease and quickness
Phosphoamino Acid Analysis of non-TCA Precipitable Proteins (Woodgett Lab)
Protein Concentration of Laemmli Gel Samples (Woodgett Lab)
-
综述
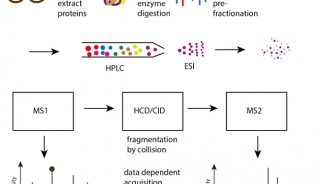
-
科技前沿