FGFR INHIBITOR, SU5402, ON CRANIOFACIAL DEVELOPMENT IN CHICKEN EMBRYOS
ABSTRACT
Fibroblast growth factor (FGF) signaling plays a signifigant role in embryonic development. Particularyl, FGFs are implicated in mediating epithelial-mesenchymal interactions by means of regulating cell proliferation and differentiation. FGF signaling is required for for limb bud and craniofacial development among other develpmental processes (Min et al. 1998; Ochuchi et al. 1997; Iseki et al. 1999; Crossley et al. 1996; Sheqetani et al. 2000). It has also been demonstrated that sonic hedgehog (SHH) signaling is necessary for proper limb bud and craniofacial development. shh null mutants exhibit impaired limb bud outgrowth, absence of digits, cyclopia and other caniofacial malformations (Chiang et al. 1996). It has been demonstrated that fgf and shh expression levels are corelated in a manner indicative of a regulatory relationship in the in developmental systems includig the limb (Gilbert 2000). To study the effect of fgf signaling on early chicken development, we interfered with FGF signaling by treating embryos with SU5402 (Calbiochem), a FGF receptor inhibitor. We observed craniofacial deformities and delayed maturation in embryos treated with SU5402. this indicates that FGF signaling is required for cranifacial formation and, more generally, development. Since FGFs are often involved in regulating shh, we processed SU5402 treated embryos with DIG-labled shh riboprobes. In situ hybridization revealed that when FGF signaling is perturbed, shh is upregulated throughout the embryo.
PURPOSE: The objective of this experiment is to study the effect of SU5402, a FGFR inhibitor, on early chicken development and the subsequent effects on shh expression.
INTRODUCTION
The development of a fertilized egg into a multicellular organism comprised of many specific tissues and structures requires a precisely coordinated repertoire of cellular processes. Cell-cell interactions are essential to the formation of an organism, and the proper genetic signals must be properly orchestrated to mediate these interactions. Paracrine factors are soluble proteins that travel short distances to induce changes in nearby cells. Mutations in genes coding for these signaling molecules can result in developmental abnormalities and in some, cases mutations are lethal.
Fibroblast growth factors(FGFs) comprise a family of paracrine factors that are involved in many developmental processes including craniofacial development, limb bud development, blood formation, and lung morphogenesis. FGFs transduce
their signal by binding to a fibroblast growth factor receptor (FGFR) which has an intracellular protein tyrosine kinase domain. The tyrosine kinase domain is activated upon FGF binding, resulting in the activation of a transcription factor by means of a signal transduction cascade (Gilbert2000). Specifically, it has been demonstrated that FGF10 signaling is required for limb bud initiation (Min et al. 1998). Also, it has been demonstrated that FGF8 is necessary to define the hindbrain and forebrain in early chick development (2000).
Another paracrine factor, Sonic hedgehog (SHH), is crucial to many developmental processes such as the dorsal-ventral specification of the neural tube, limb development, lung development, and craniofacial development. SHH functions by binding to a transmembrane receptor, Patched (Ptc). In the presence of SHH, Ptc initiates a signal transduction cascade bythat ultimately results in the transcriptional activity of a target gene (Gilbert 2000). Inhibiting SHH signaling using cyclopamine results in the cyclopia, the fusion of frontal facial processes.
Chicks were exposed to SU5402, a fibroblast growth factor inhibitor, to study the effects on development. During chick embryo development, sonic hedgehog and FGF signals work in a feedback loop which allows for development in various areas, especially the craniofacial region. Since FGF is a major chemical signal in development and closely associated with SHH, developmental defects were expected when exposed to the inhibitor. Chick eggs were injected after 2 days of incubation with a control solution, DMSO and Howard Ringer's Solution,
and an experimental 5x10-3M solution of the SU5402. One day after injection of solutions the embryos were photographed and fixed in 4% paraformaldehyde. An in situ hybridization was performed on a control and experimental embryo where they were stained for SHH. The experimental embryos were found to have defects craniofacial defects. In situ hybridization of the experimental embryos revealed increased shh expression along the notochord, limb buds, and craniofacial region. This could signify the build up of SHH due to the interference of FGF signaling. The results reinforce the importance of FGF signals in chick development and the ability of SU5402 to interfere with FGFRs.
PROTOCOL
1. Incubate chicken eggs for 2 days.
2. Remove eggs from incubator. Aseptically window eggs(do not remove membrane) and inject control and experimental eggs with 1uL of solution into yolk and away from embryo.
a. Control egg solution: 1mL of Howard Ringer's solution, 100uL DMSO (5
mM)
b. Experimental egg solution: 1mL of Howard Ringer's Solution, 100 uL
SU5402* (5 mM).
*handle SU5402 with extreme caution!
3. After windowing and injecting the control and experimental solutions, place in 37ºC incubator for 1 or 2 day(s).
4. Open eggs and remove embryo aseptically. Clean embryos in Howard Ringer's Solution and capture images of experimental and control embryos.
5. Preserve in 4% Paraformaldehyde.
6. Process embros with DIG-labled shhriboprobe formin situhybridization.
7. Capture images ofin situhybridized experimental and control embryos.
RESULTS
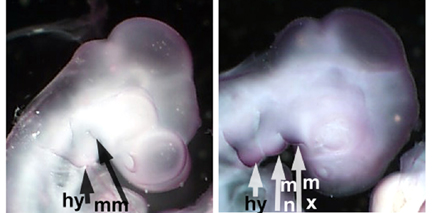
FGF signaling is crucial to proper embryonic development. To determine how interfering with FGF signaling affects 2 day embryos we treated embryos with SU5402, a FGFR inhibitor. The survival ratio for control and experimental embryos was 87% (n=16) and 58% (n=24), respectively, when incubated for 1 day after injection. Interestingly, the experimental embryos exhibited normal limb bud development. However, the craniofacial region of some of the treated embryos were malformed in that they exhibited an abnormal head to body ratio(head being smaller than normal). The most prominent defects were reduced size forebrain, midbrain, and frontal-nasal process (Figure 1). We also observed abnormal blood pools, indicative of poor vascularizaation. The control embryos were developing normally and had no noticeable malformations, when they were observed and photographed (Figure 1).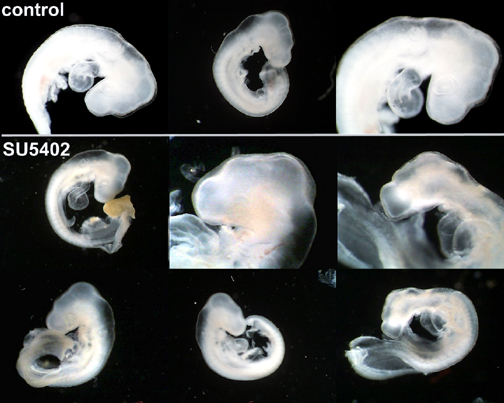
(a)
(b)
Figure 1. SU5402 treatment. Experimental embryos display delayed development
and craniofacial defects. (a) Darkfield images: control- first row, SU5402 - bottom two rows; (b) Digital images: SU5402 - top row, control - bottom row
Embryos incubated for 2 days after SU5402 injection showed a 16% (n=12)survival rate. Surviving experimental embryos displayed severe cases of the craniofacial deformities mentioned above as well as extensive blood pooling at the posterior end of the embryos (Figure 2). The survival rate for the control embryos was 86% (n=6). Since SHH and FGF are known to be involved in regulatory feedback loops in the the limb, lung, feather bud and other systems we processed embryos with shhriboprobe to determine the effect of interfering with FGF signaling on levels of shh
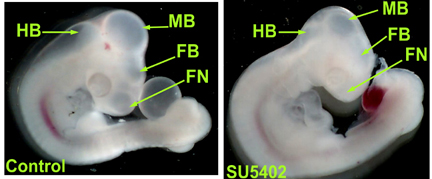
Figure 2. Comparison of control and SU5402 treated embryos. SU5402 results in
decreased hindbrain (HB), midbrain (MB), forebrain (FB), and frontal nasal (FN) processes.
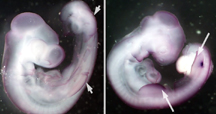
expression. The experimental embryos exhibited a greater amount of shh staining in both the limb buds and craniofacial region than the control embryos. In the limb buds the domain of shh expression extended to the anterior portion of the limb bud, whereas in the controls shh was confined o the posterior portion of the limb buds. Both the control and experimental embryos show staining along the notochord (Figure 3).
|
|
(a)control/shh (b)SU5402/shh
Figure 3. Embryos processed with DIG
labled shh antisense probe. (a) embryo
treated with DMSO exhibits less shh
expression (b) interfering with FGF
signaling resulted in greater shh
expression in the craniofacial and
limb bud regions(white arrows) as
well as the branchial arches.
DISCUSSION
Genetic modification of mice embryos to produce mice deficient for Fgf10 lacked limb buds. This revealed that FGF10 is necessary for limb bud initiation. Treating embryos with SU5402 (final concentration 5 uM) did not inhibit limb bud outgrowth. This lead us to conclude that we did not completely inhibit FGF signaling and/or FGFR activity was restored some point after treatment before limb bud initiation. A possible way to remedy this result would be to reinject embros after the initial treatment or to treat the embryos at a time crucial to limb bud initiation.
It has been demonstrated that mesodermal Fgf-10 initiates limb bud outgrowth and initiates the expression ofFgf8in the AER which subsequently upregulates Shhin the posterior margin or the developing limb bud. Subsequently, SHH upregulates Fgf4in the AER which in turn positively regulates Shh in the posterior limb. Furthermore, Sanz-Ezquerro and colleagues have demonstrated that SHH participates in an auto regulatory feedback loop in the limb bud (2000). When they ectopically expressed shh, they found that shhwas subsequently downregulated (Sanz-Ezquerro et al. 2000). As indicated by the high level of shh expression in the limb buds, we suspect that the FGF inhibition interfered with the autoregulatory mechanism of SHH (Figure 4).
Previous research indicates that mutations in FGFR1, FGFR2, and FGFR 3 result in cranial malformations that involve multiple or all sutures (Wilkie 1997; as cited by
Iseki 1999). It is also known that FGF8 signaling is necessary for the proper separation of the midbrain and hindbrain (Shigetani2000). Embryos treated with SU5402 were morphologically different from the control embryos primarily in the craniofacial region. The size of the experimental embryo's brain sections were smaller and narrower in shape than in the controls. We attribute the observed malformations to the interference of FGF signaling and conclude FGF signaling is necessary for proper craniofacial development.
In the craniofacial region, FGF is known to signal Bmp4which in turn downregulates shh (Cebra-Thomas, personal communication). According to our results for the in situhybridization, we propose that FGF signaling is reduced by SU5402, then Bmp4is also reduced which leaves a minimal amount of signaling factors to downregulate the shh. The control embryos which were processed for in situhybridization with a shhantisense probe have blue outlines of the the craniofacial region indicating shh expression. The SU5402 treated experimental embryos, which underwent the same procedure and staining, had an excess of blue staining in the craniofacial region,particularly the anterior midbrain. This supports the conclusion that the feedback loop of fgf and shh was broken, and that there was severely reduced ability to downregulate shh. If the loop was still functioning, then staining in the craniofacial region of the experimental embryos would have been present but in a reduced and controlled manner. Results show the craniofacial region and limb bud region of chicken embryos as
the area most effected by SU5402 and its reduction of FGF signaling. Other regions are likely to have been effected due to the strength of SU5402, but there was no conclusive evidence for these defects. Results could have been dependent on time of treatment administration, dosage size, and repetition of treatments. It is possible that at different times of treatment could have effected various developmental processes. The amount of dosage could have had an effect on noticeable defects and repetition of dosages could have caused greater damage by not allowing the chicken embryos to recover.
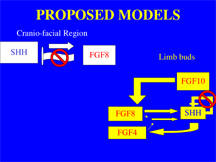
A .............................B
Figure 4. Proposed Models. A)In the craniofacial region SHH induces fgf8 which subsequently downregulates shh. We propose that interfering with FGF signaling resulted in increased levels of shh expression in the craniofacial region. B) We propose that interfering with FGF signaling resulted in failure of the shh autoregulatory mechanism and thus over expression ofshh in the limb buds.