Regulative Development in Axolotl Embryos; Splitting the Heart Field
Objective
This experiment will seek to demonstrate regulative development in Ambystoma mexicanum embryos. Specifically, we will split the morphogenetic field that is responsible for heart formation using grafted tissue from the gill area, and attempt to form two hearts – one on either side of the grafted tissue.
Background
Organisms that use regulative development rely on cell-cell interactions to determine what develops where, rather than having fixed cell fates (as in mosaic development). Because of this, embryos that undergo regulative development are able to compensate for missing or damaged parts (Gilbert, 2003). This sort of compensation is particularly seen in regions known as morphogenetic fields. These regions of cells are each committed to form a certain organ, but made up of individual cells that are not yet committed to form a specific part of that organ. Damage to these fields can be compensated for by the other cells in the field (Gilbert 2003).
In the axolotl salamander (Ambystoma mexicanum), the heart forms through the fusion of two regions of heart forming tissue -- the heart primordia (Hamburger, 1973). In order for the axolotl to have one fully formed heart, these two regions must communicate with each other so that they can "decide" when and how to come together. Theoretically, if this communication were interrupted by damage to the tissue (or a barrier placed between the primordia tissue), the heart primordia would be unable to fuse into one. This communication and subsequent ability to compensate for damage, is a prime example of regulative development.
This experiment will take advantage of the morphogenetic field of the axolotl heart. The fact that two hearts form is not only amazing to behold, but demonstrates that the cells, when prevented from communicating with each other, will go on to form two separate organs. This is a direct result of regulative development.
Materials
Axolotl embryos (stage 14-16)
1% agarose-coated operating dishes
100% HEPES-buffered Modified Steinberg's Solution (HBSt) with antibiotics (gentamycin at 70 mg/ml)
50% HBSt
20% HBSt
Microsurgery tools (tungsten knives and hair loops)
Procedure
1) Remove the first two jelly coats from several stage 14-16 embryos
a. Transfer embryos into dish of 20% HBSt using plastic pipet (the tip should be cut off so that you can suck up the embryos as if they were the bubbles in bubble tea).b. With finest forceps, grab and pierce the more interior of the two visible jelly coats. This takes practice and patience, as the jelly is very slippery.
c. Once pierced, firmly pull the jelly coat apart, taking care not to squash the embryo in the process. The embryo should pop out nicely once the jelly coats are opened up enough.
2) Remove the vitelline membrane
a. Prepare 1% agarose-coated operating dishi. Heat glass pipet tip using Bunsen burner until melted into a little ball at the tipii. Press hot tip to several places in the dish to form little indentations in the agarose. This will help hold the embryos in place while operating.
iii. Fill dish with 100% HBSt with gentamycin
b. Place embryos in operating dish and let sit for about 5 minutes. This will allow the vitelline membrane to puff up with water somewhat, making it easier to remove.
c. With plenty of light from the stereoscope, look for vitelline membrane around embryo. Gently try to grab it with fine forceps and pull off. Again, this takes practice and patience. * Damaged embryos should be transferred to a separate dish for use as tissue donors etc.*
3) Obtain gill tissue from stage 16 donor
a. Place donor embryo in one of the indentations in the dish with its flank side facing up, ventral side toward you, and dorsal away.b. In the lower anterior corner there should be a slightly lighter colored region (the presumtive gill tissue); this is what you want to cut out (Figure1); use a tungsten knife to cut and a hair loop to help hold the embryo in place. This cutting takes some getting used to; don't be surprised if at first you end up squishing the embryo into many little yolky cells.
4) Prepare host (~ stage 15) for tissue transplant
a. Place host embryo in one of the indentations with ventral side up.b. Using the tungsten knife to cut and hair loop to help hold the embryo in place. Make an incision that is about a third the length of the embryo, but towards the anterior. You just want to break the exterior and scrape a little way into the cells so there will be enough room for the graft. This also takes a bit of practice.
5) Put tissue transplant into host
a. Pick up tissue transplant with hair loop and transfer into incision in host.b. Use tungsten knife and hair loop to gently but firmly push tissue into incision
6) Allow to heal overnight in their operating dishes, then gradually replace solution to reduce concentration to 20% HBSt.
7) After embryos heal, transfer to fresh agarose-coated dish.
8) After 3 or 4 days, check for heart pulsations, being sure to note if two heats have formed, and if they have, whether or not they beat in synch.
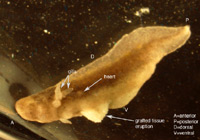
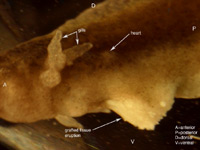
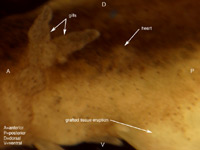
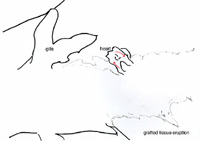
Six axolotl embryos were initially prepared successfully, and of those, at least one showed a distinct heart beat by 6 days after surgery. Determining whether or not the heart field had been affected by the graft was difficult due to the opacity of the axolotl skin, and the positioning of the embryo in the operating dish; one side down, such that only one side could be viewed at a time without damaging the embryo. Evidence that the heart field was not particularly affected includes the fact that the grafted tissue was at least partially rejected. Additionally, when viewing the gills close up, the flow through them seemed completely synchronous between the left and right gills, as well as in synch with the visible heart beat. As for the stage of heart development, it appears to have developed at least somewhat normally to a point somewhere between stages A and B in Figure 7. Specifically the blood flow seemed to start toward the ventral-anterior corner of the heart, flow upwards toward the dorsal area, and finally down to the ventral-posterior region (Figures 5 and 6). There were some irregularities however. In Figures 3, 4, 5 & 6 we can see that the heart region is well behind the gills. This is somewhat farther back and more to one side than one would normally expect to find the heart. This irregularity opens up the possibility that the heart field was at least somewhat affected, despite appearing to have a synchronous heart beat. Figure 3. Photo of six day axolotl embryo showing location of heart and early heart development. Note eruption of previously grafted tissue from ventral region. Figure 4. Close up of figure 3, focusing on heart region and tissue eruption. Figure 5. Close up of figure 3&4, focusing on heart region. Figure 6. Outline diagram of figure 5, showing specifically the outline of the heart region. The red arrows indicate the observed direction of blood flow. A B Figure 7. Two early stages of amphibian heart development; heart development in above figures 3-6 is presumed to be between these two stages. Diagrams simplified after Rugh, 1951. From our observation of a single distinct heartbeat, it appeared that the attempt to divide the heart field was not successful. Additionally the observed direction of blood flow and apparent synchronous flow through the left and right gills seemed consistent with normal amphibian heart development (Rugh, 1951). However, the ability to observe the heart beats was hindered by the opacity of the axolotl skin, as well as the position of the embryo in the operating dish, so these observations may not accurately reflect the true condition of the heart. In addition, the heart appeared to be somewhat more posterior than would normally be expected. The presence of this irregularity leads us to believe that the heart field was affected in some way. If this were the case, the heat could actually be split, or partially split, but have two heart beats that happen to appear synchronous.
9) Collect photos and video of successful hosts.Results
Discussion
The fact that the grafted tissue was observed erupting from the belly/ventral region of the embryo may suggest that the graft, which was intended to split the heart region, was not incorporated into the embryo enough to sufficiently block communication between the two halves of the heart field (Figures 3, 4,&5). It should be noted that there are other explanations for these data that might also be evidence for regulative development. For instance, the gill tissue could be transforming into heart tissue through cell-cell interactions. However, the relatively advanced developmental stage of the donor tissue, and the fact that the photos seem to clearly show tissue rejection, do not support this conclusion. In the end, these results are at best inconclusive in regards to regulative development in the formation of the axolotl heart.
It should be noted however, that previous experiments using small pieces of sterile foil as a barrier rather than gill tissue, did yield results supporting the existence of regulative development in the axolotl heart. Specifically, two hearts, one on either side of the foil barrier, were seen to develop and clearly beat asynchronously (Vélez and Krsmanovic, 2004). Other examples of the manipulation of morphogenetic fields, and therefore evidence of regulative development, have been shown in limb regeneration studies. Axolotls are actually able to compensate for damage to the limb morphogenetic fields throughout their lives, to the point where entire limbs may be regenerated from the remaining tissue of a limb stump (Gardiner et al 2002).
Since studies show that there is ample evidence for regulative development in axolotls it would therefore seem that the our results likely have more to do with experimental technique than absence of regulative development in axolotls. While this tissue graft procedure was not particularly complex, and was the method originally suggested in Hamburger's Manual of Experimental Embryology, in retrospect it does not seem well suited to those who have had little to no practice doing microsurgery. Additionally, it should be noted that while the embryos were largely at the appropriate stage at the beginning of the procedure, as time went on, the warmth of the room allowed them to develop more quickly than we had anticipated, leading to fewer suitable embryos. It is important to remember that there is only so much time before the heart primordia fuse, and grafts must be done before this happens in order to be sucessful. In the future it would be advisable to keep the embryos cold whenever they are not actually being worked on, rather than letting a number of them sit out at room temperature.