Interleukin-6 Induced Acute Phenotypic Microenvironment Promote...(十)
In this study, we found that “acute” response was strongly induced by cryo-thermal therapy with “acute” and high expression of a series of acute phase proteins and consequently reverse the tumor chronic pro-tumorigenic environment. “Acute’ response is generally occurred as an immediate and evident inflammation in response to stress. In tumor progress, chronic environment is often induced with little visible inflammatory reaction, whereas it becomes apparent and severe upon extensive tumor expansion [73]. As the critical upstream regulator of acute response, IL-6 was also identified with an “acute” profile in our study, whose reduced level decreased the magnitude of “acute” response. Previously, “acute” IL-6 induced by adipose-derived stromal/stem cells has been proved to mediate the good therapeutic effects in acute lung injury [74]. In addition, fever-range thermal stress induced “acute” activation of IL-6 has been indicated with anti-tumor activity through boosting T lymphocyte trafficking [75-77] and amplifying the killing cytotoxic effector of CD8+ effector T cells toward tumor cells [78]. However, the sustained elevated IL-6 concentration in circulating has been observed in numerous cancers and is a prognostic indicator of poor outcome [79-81]. Hence we hypothesized that “acute” IL-6 induced “acute” response plays a key role in anti-tumor activity.
In this study, we proposed a model that IL-6 induced “acute” response could mediate anti-tumor activity in three aspects, which could interact with each other and result in the good therapeutic efficacy. They are 1) Th2 immunosuppressive response breaking, shifting pro-tumorigenic phenotype toward 2) tumoricidal innate and Th1 adaptive immunity activation and 3) up-regulation of tumor progression and metastatic inhibitors. The details were described below (Figure 14).
Cryo-thermal therapy, by applying through rapid freezing followed by heating, would result in severe thermal and mechanical stresses to local tumor. The severe stress could thus stimulate a rapid and vast generation of IL-6 through macrophages influx. Such “acute” activation of IL-6 rapidly induces the “acute” phase response, generating an “acute” microenvironment, and disrupting the chronic inflammation in tumor site. Such “acute” microenvironment restricts the expression of ICOSL around the baseline, well controlling the Th2 immunosuppressive response (like Tregs, IL-4, IL-5, and IL-13) induced by chronic environment and favoring a protective immunity hub. Under this “acute” environment, acute phase proteins (APPs) are up-regulated, facilitating the phagocytosis of tumor cells and clearance of injurious agents through opsonisation or secreted pathogen recognition receptors [36, 82]. In addition, complement system is also activated, further improving the clearance of damaged tumor cells through phagocytosis, and facilitating the lysis of invading tumor cells via the formation of the terminal membrane attack complex (MAC) [83] to release the metastatic risk. By such manner, anti-tumor innate immunity is initiated. Subsequently, complement system could drive the innate toward adaptive immunity to promote the anti-tumor immune response. It has been shown that its products, such as anaphylatoxins (C3a, C5a) [84, 85], C1q and C3 fragment [86-88] can directly induce dendritic cells (DCs) maturation and promote the development of effector T cells. This is in line with our observation that CTSL was up-regulated and more DCs were maturated on the similar day to complement system activation (3rd and 5th day). In addition, it has been reported that “acute” IL-6 could limit the activity of virus-specific Tregs, thereby facilitating the activity of virus-specific memory CD4+ T cells[89]. Consistently, in our study, an increased number of CD26+CD4+ memory T lymphocytes were activated and proliferated accompanied by the“acute” response, favoring the Th1 anti-tumor adaptive immunity. To be noted, in our study, activated memory T cells appeared a bit earlier than the matured DCs augmentation (2nd day vs 3rd day). In fact, memory T cells have lower need of MHC-peptide for activation than naïve T cells and response more fast and robust [90]. Such rapid response allows memory T to attack the tumor cells as early as possible. Furthermore, DC producing IL-12 contributes to the development of Th1 that generates high amount of IFN-γ to further promote Th1 polarization, while suppressing Th2 cell differentiation, favoring the protective Th1 adaptive immune response. Notably, “acute” response started to resolve with reduced production of IL-6, declined expression of APPs on 2nd day and less leukocyte trafficking. Such resolution is critical, not only to maintain the homeostasis of hostile environment, but also bridge the gap between innate and adaptive immunity for the optimal development of adaptive immunity [91].
What’s more, “acute” micro-environment activated the secretion of tumor progression and metastatic inhibitory proteins by host cells to reduce metastasis. Finally, with the struggle of above three aspects, “acute” response induced by cryo-thermal therapy switches the Th2 immunosuppressive, pro-tumorigenic environment to a Th1 immunostimulatory, tumoricidal phenotype, protecting the host from accumulated tumor burden, reducing metastasis and recovering the host metabolism in the end.
To be noted, APPs studied in our work are relative high abundant proteins, which benefit their identification and quantification in cryo-thermal therapy and other biological events, like in cryotherapy or hyperthermia treatment of cancer. However, their “acute” profiles are markedly different. Cryo-thermal therapy induced the strongest “acute” response and elicited the most pronounced immunological effect compared to cryotherapy and hyperthermia alone. Moreover, cryo-thermal therapy on healthy tissue induced weaker “acute” response and failed to stimulate effective immunological effect compared with tumors. In this case, we suggested that the magnitude of “acute” and the release of “danger” signals play a key role in determining the efficacy of anti-tumor immunity.
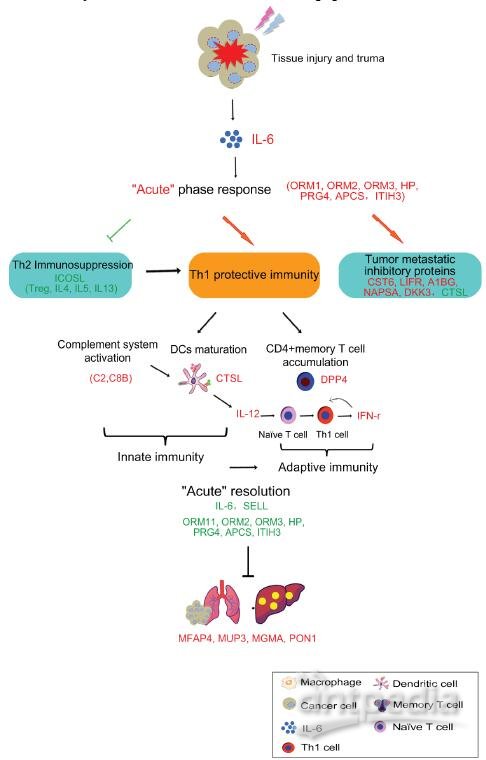
Figure 14. The schematic graph of “acute” response mediated anti-tumor activity induced by cryo-thermal therapy. The cryo-thermal therapy performed by rapid switch between freezing and heating causes a severe thermal and mechanical stresses to local tumor, leading a rapid and vast generation of IL-6 by macrophage influx. The “acute” IL-6 induced “acute” response could mediate anti-tumor activity in three aspects, which could interact with each other and enhance the anti-tumor effect. They are 1) Th2 immunosuppression breaking, 2) driving anti-tumor innate and Th1 adaptive immunity activation, characterized by complement system activation, more matured DCs and CD4+ memory T cell accumulation, as well as the secretion of Th1 cytokines to promote the differentiation of naïve T cells to Th1 cells, and 3) up-regulation of tumor progression and metastatic inhibitory proteins. In addition, “acute” resolution is critical, not only to maintain the homeostasis of hostile environment, but also bridge the gap between innate and adaptive immunity for the optimal development of adaptive immunity. Finally, “acute” response mediated anti-tumor activity leads to the recovered liver metabolism and normal physiological function. In addition, tumor recurrence is reduced and metastasis is inhibited. Proteins in red are up-regulated, green are down-regulated.