Interleukin-6 Induced Acute Phenotypic Microenvironment Promote...(六)
Cryo-thermal therapy induced an “acute” phase response
First, we performed PRM analysis upon significant proteins involved in acute phase response, namely APCS, HP, ITIH3, ORM1, ORM2, ORM3, and PRG4, and they are defined as acute phase proteins (APPs). ORM2 and HP were quantified with an identical significant pattern as shotgun proteomics. Proteins such as ORM1, ORM3, APCS, ITIH3 and PRG4 were found to be significantly regulated at more time points in PRM analysis (Additional File 6: Table S6, Figure 5). These proteins revealed a remarkable difference between treated and control mice. In particular, in control mice, these proteins persistently expressed at a significantly higher level than healthy mice and rapidly elevated to peak during the late stage of tumor development. A similar high expression was observed in treated mice 2 h post therapy, however, such expression peaked on the 2nd day, much higher than that of the control mice. Notably, the peak expression was transient (Figure 5, Additional File 7: Figure S6A). Although termed as “acute”, such response could turn into chronic upon persistent stimuli [36]. In this case, the persistent expression of APPs indicated that mice without treatment were involved in a long lasting inflammatory environment during tumor development. By contrast, cryo-thermal therapy rapidly drove a much stronger, but “acute” inflammatory response, breaking the chronic environment. Additionally, chronic inflammation is characterized by the disrupted leukocyte trafficking at inflamed sites [37]. In our study, SELL, an adhesion molecule mediating the leukocyte trafficking from bloodstream to inflamed sites [38, 39], was quantitatively analyzed using PRM, resulting in a similar pattern to shotgun proteomics (Additional File 6: Table S6). Compared to healthy, it constantly stayed at a much higher level in untreated mice (Figure 5), suggesting the persistent accumulation of inflammatory cells within tumor, and supporting the chronic inflammation mentioned above. By contrast, SELL was significantly down-regulated and gradually reduced to the base level during the late time points post therapy (Figure 5). This finding was consistent with the observed resolution of acute phase response in treated mice.
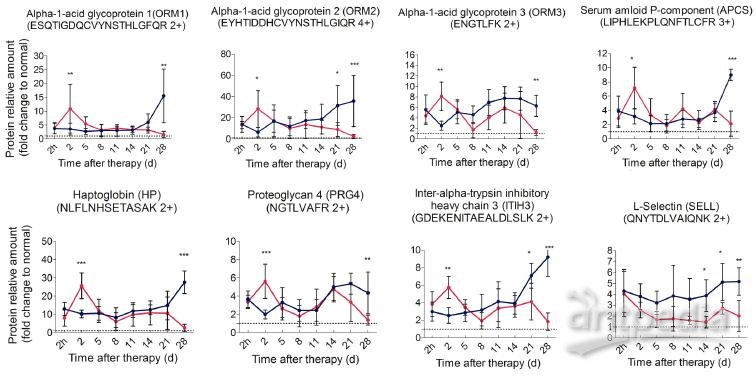
Figure 5. Time course verification of proteins associated with acute phase response and leukocyte activity using parallel reaction monitoring. Individual peptide’s abundance was used as a proxy of protein’s abundance. Peptide’s abundance in mice treated and untreated was divided by its corresponding partner in healthy mice and plot over time. The dash line represents the protein expression level in healthy mice. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 by two-way ANOVA with the Bonferroni correction. n=5~6 for each condition, except that on the 28th day, only 3 mice left in the control group.
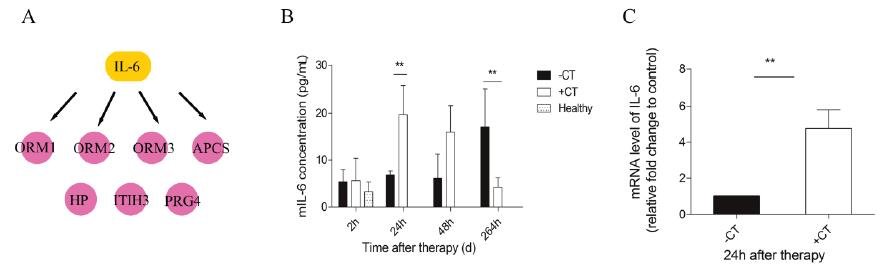
Figure 6. IL-6 was elevated in serum and local tumor site in a short time after therapy. (A) IL-6 was identified as the upstream regulator of acute phase proteins. (B) The amount of IL-6 in serum was measured by ELISA. (C) The expression of IL-6 within tumors was examined with RT-PCR. Data are shown as mean ± SD. **p < 0.01, by (B) two-way ANOVA with the Bonferroni correction; n=3 with two replicates assay. (C) Student t test. Triple replicates assay.
“Acute” interleukin-6 was induced by cryo-thermal therapy
Next, we carried out a network analysis on these APPs using IPA. Interestingly, interleukin-6 was identified as the key upstream regulator of these proteins (Figure 6A), indicating its significant role in inducing acute phase response. To further confirm this assumption, first, IL-6 levels in serum and within tumor were examined via ELISA and RT-PCR. As a result, IL-6 in circulation maintained at a persistent high level in control mice relative to healthy and even extensively increased on the 11th day (Figure 6B). However, cryo-thermal therapy immediately stimulated a considerable expression of IL-6 in serum and within local tumor on the 1st day, much stronger than mice untreated (Figure 6B, Figure 6C), whereas started to decline on the 2nd day and returned to a similar level as the healthy mice (Figure 6B). These findings are consistent with the APPs expression profiles. Next, prior to its peak expression, we neutralized IL-6 with IL-6 antibody in vivo 3 and 24 h post therapy. Serum was collected 2 days post therapy to examine the expression of APPs. Among APPs, ORM, APCS and HP are well documented in researches [40-42]. In addition, ORM1, ORM2 and ORM3 belong to the same family and ORM1 was identified with the most confident peptides in shotgun and PRM target proteomics compared to ORM2 and ORM3. In this case, ORM1, APCS and HP are selected. Western blot analysis showed that their expressions are significantly down-regulated after IL-6 neutralization (Figure 7). All these results indicated that “acute” activation of interleukin-6 was stimulated by cryo-thermal therapy, inducing the “acute” phase response.
“Acute” response could temper the Th2 immunosuppressive environment
As described, with the accumulating tumor burden, the expression of APPs steeply raised to the most on the last day (Figure 5), indicating the most severity of chronic inflammation. Chronic inflammation is associated with immunosuppression in many tumors [43], predominately induced by tumor infiltrating Tregs to suppress effector T cells and prevent tumor against immune surveillance [44]. The interaction of ICOS /ICOSL can boost Tregs activation, proliferation, and survival [45-47]. In addition, ICOSL could trigger the expression of immunosuppressive Th2 cytokines, such as IL-4, IL-5 and IL-13[48, 49]. Here, both shotgun proteomics discovery and PRM validation analysis revealed that ICOSL was significantly overexpressed during the late stage of tumor development, whereas it always stayed around the base line under “acute” response (Figure 8). This observation suggested that the Th2 immunosuppression may be attenuated by the cryo-thermal therapy induced “acute” response and thus the activation of anti-tumor immunity was favored.
“Acute” response could elicit innate and Th1 adaptive anti-tumor immunity
Since “acute” is a remarkable profile of cryo-thermal therapy, which leads to the attenuation of immunosuppression, we then interested in how this “acute” signature could benefit the anti-tumor immunity. We further evaluated proteins significantly expressed in the timeframe of “acute” response (2nd to 5th day post therapy) in shotgun proteomics. These proteins include CPB2, CTSL, C2, C8B, CFP, DPP4, MUP3, GRM7, VNN3 and VASN. Interestingly, among them, C2, C8B, CFP are involved in the activation of complement system; CTSL and DPP4 are responsible for T cell activation. All of them appear to regulate innate and adaptive immunity. Hence, we performed PRM target analysis to further examine these proteins. As a result, the significant expression of C2, C8B and CTSL, DPP4 were well in line with the shotgun proteomics (Table S6).
Particularly, C2 is the central regulator of classic and lectin complement pathways, and C8B is the component of terminal membrane attack complex (MAC) for cell lysis. These two proteins were reported to play critical roles in tumor cell clearance through phagocytosis and cell lysis, triggering innate immunity [50, 51]. In consistent, both proteins were shown being significantly up-regulated on the 5th day after treatment and returned to the base line, which is in agreement with the “acute” response profile. On the other hand, under chronic inflammation in untreatedmice, these proteins were shown to stay around the base level (Figure 8). All these observations implied that “acute” phase response could re-activate the impaired complement system under chronic inflammation and benefit anti-tumor innate immunity.






















