Interleukin-6 Induced Acute Phenotypic Microenvironment Promote...(九)
The strongest and unique “acute” environment induced by cryo-thermal therapy stimulated the most effective anti-tumor immune response
As described above, “acute’ environment is suggested to be the key driver to switch tumor microenvironment from immunosuppressive to immunostimulatory state. In addition, the combination of cryo/hyperthermia showed prolonged survival rate and reduced metastasis compared with cryotherapy or hyperthermia alone. In this case, we are interested in the “acute” profile in different therapeutics and the relationship with anti-tumor immunity. First, we compared the expression of three well documented APPs (ORM1, APCS and HP) [40-42] in serum 2 days post different treatments on tumors, which are 1) cryo-thermal therapy, 2) cryotherapy, 3) hyperthermia and 4) control without treatment. Western blot analysis showed that cryo-thermal therapy induced the strongest acute response as indicated by the highest expression of selected APPs (Figure 12). As described above, “acute” response could lead to DC maturation and Th1 adaptive immune response in early stage. To better compare the immunological effect stimulated by “acute” response, we next tested the adaptive immune response in later stage. As a result, cryo-thermal therapy elicited a significant protective immune response with the most accumulated CD8+ and CD4+ T lymphocytes in spleen 14 days post treatment (Figure 13A, 13B). In addition, the immunosuppressive state was most inhibited indicated by the maximally reduced MDSC (Gr1+CD11b+) (Figure 13C). These findings suggested that as a combination of cryo/hyperthermia, cryo-thermal therapy established the strongest “acute” environment, which could benefit the stimulation of the most pronounced anti-tumor adaptive immune response. Moreover, to delineate the "unique" immunological effect of cryo-thermal therapy on tumor tissues, we further examined the “acute” profile and corresponding immune response in heathy mice under this specific therapy. As shown, cryo-thermal on healthy mice (N+A) induced “acute” response; however, such “acute” pattern was much weaker than that applied on tumors (Figure 12). We assumed that the “danger” signal released from tumors could boost the host “acute” inflammation. In addition, cryo-thermal therapy induced the most pronounced protective T lymphocyte response on tumors, whereas it was not changed much in healthy mice, regardless of treatment or not, indicating that such immune response was not effectively stimulated without the presence of antigen released from tumors (Figure 13A, 13B). As shown, MDSC, with remarkable immunosuppressive abilities in cancer, was largely reduced after cryo-thermal therapy on tumors; however, it was induced under “acute” response in healthy mice, which could quickly differentiate into mature myeloid cells predominantly participating in innate immune response, while have a minimal impact on the adaptive immune response [72] (Figure 13 C).
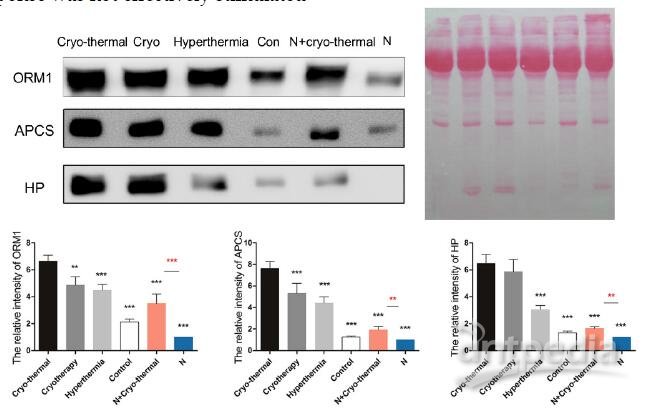
Figure 12. The expression of acute phase proteins through different energy-based therapeutics applied on tumors and healthy mice. Tumor bearing mice were treated with 1) cryo-thermal therapy, 2) cryotherapy, 3) hyperthermia and 4) control (no treatment). Healthy mice with and without cryo-thermal were set as another controls. 2 days post therapy, serum was collected for western blot analysis. Protein amount was evaluated and visualized with Ponceau S. Blots were evaluated with Quantity One 1-D. Results are expressed as relative pixel intensity normalized with healthy group. Data are shown as mean ± SD. **p<0.01, ***p < 0.001 by one way ANOVA with Dunnett's test and each group was compared with cryo-thermal therapy. Healthy mice with and without treatment were analyzed by student t test. n=3 with three technical replicates.

Figure 13. The protective adaptive immune response and immunosuppression arising from different energy-based therapeutics applied on tumors and healthy mice. Tumor bearing mice were treated with 1) cryo-thermal therapy, 2) cryotherapy, 3) hyperthermia and 4) control (no treatment). Healthy mice with and without cryo-thermal were set as another controls. Lymphocytes were isolated from spleens 14 days post therapy. (A) The percentage of CD3+CD4+ T lymphocytes. (B) The percentage of CD3+CD8+ cytotoxic T lymphocytes. (C) The percentage of Gr1+CD11b+ MDSC cells. Data are shown as mean ± SD. *p<0.05, **p<0.01, ***p < 0.001 by one way ANOVA with Dunnett's test and each group was compared with cryo-thermal therapy. Healthy mice with and without treatment were analyzed by student t test. n=3.
Taken together, we concluded that cryo-thermal therapy has a better immunological effect over cryo/hyperthermia alone and such effect was unique on tumors.
Discussion
Metastatic breast cancer remains a big challenge in clinical practice. Based on thermal therapeutic strategy, we previously developed the cryo-thermal therapy as an energy-based cancer intervention. This strategy has shown a good therapeutic efficacy with prolonged lifespan and reduced metastasis (Figure 1). In this study, serum glycoproteome comparison using shotgun proteomics analysis followed by PRM target validation was carried out to dissect the distinct protein profiles between treated and untreated mice on multiple samples (94) and time points (8), in order to better understand the molecular mechanism of the anti-tumor activity on a system-wide view. In general, targeted proteins exhibited consistent significant expression with that in shotgun proteomics, to some extent, the enhanced sensitivity and selectivity of PRM assay provides better quantitative information for some proteins, such as ORM1, ORM3, APCS, ITIH3, PRG4, SELL, MUP3, MGAM and MFAP4. More importantly, such high throughput target proteomics allowed us to validate proteins on a large scale (multiple samples and proteins), which was not suitable using traditional approaches such as western blot and ELISA, due to limitations from time-consuming and antibody dependence.
-
科技前沿

-
焦点事件