AbC™ Anti-Mouse Bead Kit
实验概要
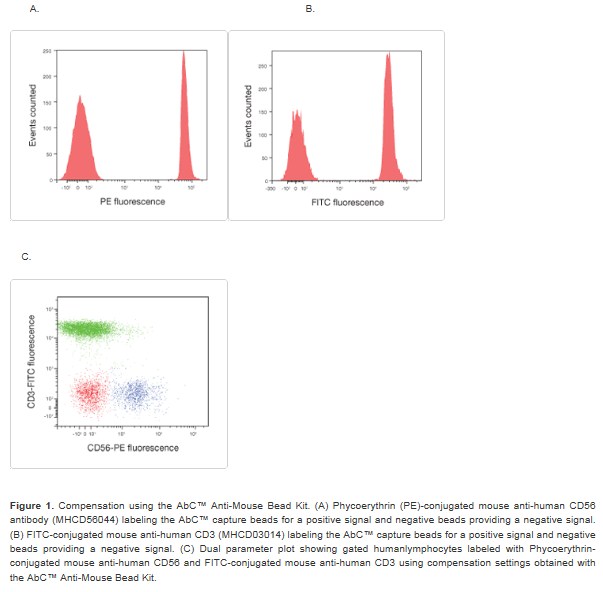
The AbC™ Anti-Mouse Bead Kit provides a consistent, accurate, and simple-to-use technique for the setting of flow cytometry compensation when using fluorochrome-conjugated mouse antibodies (Figure 1). The kit contains two types of specially modified polystyrene microspheres, the AbC™ capture beads, that bind all isotypes of mouse immunoglobulin, and the negative beads that have no antibody binding capacity.
After incubation with a fluorochrome-conjugated mouse antibody, the two bead components provide distinct positive and negative populations of beads that you can use to set compensation. You can perform compensation with the same fluorochromelabeled antibody used for cell staining. Because of the consistent nature of bead scatter and high surface antibody binding capacity, this allows you to more consistently and accurately set compensation for any combination of fluorochrome-labeled mouse antibodies.
主要试剂
The AbC™ capture beads and negative beads have a diameter of approximately 6 μm (actual size for each lot is listed on the component vial). The bead suspensions are supplied in dropper vials for convenient sample application.
Table 1. Contents and storage information.
Material | Amount | Composition | Storage* | Stability | |
AbC™ capture beads (Component A) | 5 mL | 3 × 106 beads/mL in phosphate buffered saline (PBS) with 0.1% BSA and 2 mM sodium azide |
| When stored as directed, this kit is stable for at least 1 year. |
Number of assays: Sufficient material is supplied for 100 assays based on the protocol below.
Materials Required but Not Included:
Mouse antibody conjugate
PBS or equivalent
实验步骤
1. Using the AbC™ Anti-Mouse Bead Kit
1)Completely resuspend the AbC™ capture beads (Component A) and negative beads (Component B) by gently vortexing for 30 seconds before use.
2)Label a sample tube for each fluorochrome-conjugated mouse antibody you are using, and add 1 drop of AbC™ capture beads (Component A) to each tube.
3)Add a pre-titrated amount of each mouse antibody conjugate to the AbC™ capture bead suspension in the designated tube and mix well. Make sure to deposit the antibody directly to the bead suspension.
4)Incubate for 15 minutes at room temperature, protected from light.
5)Add 3 mL of PBS or other buffer to sample tubes. Centrifuge for 5 minutes at 200 × g.
6)Carefully remove the supernatant from tubes and resuspend the bead pellet by adding 0.5 mL of PBS or other buffer to sample tubes.
7)Add one drop of negative beads (Component B) to the tubes and mix well.
8)Vortex tubes before analyzing using flow cytometry. You may briefly sonicate to increase the percentage of singlet beads, if necessary.
9)Perform manual or automatic compensation according to the preferred procedure for the flow cytometer in use. Gate on the bead singlet population based on FCS and SSC characteristics.
2. Combining AbC™ and ArC™ Kits
The ArC™ Amine Reactive Compensation Bead Kit is designed to facilitate compensation when using any of the LIVE/DEAD® fixable dead cell stains, all amine-reactive dyes. This kit provides two types of specially modified polystyrene microspheres, the ArC™ reactive beads (Component A) that bind any of the amine-reactive dyes, and the ArC™ negative beads (Component B), that have no reactivity. Using the two kit components with any aminereactive dye will provide distinct positive and negative populations of beads. You can use the AbC™ Anti-Mouse Bead Kit and the ArC™ Amine Reactive Compensation Bead Kit together to calculate compensation in multicolor immunophenotyping experiments that incorporate a LIVE/DEAD® fixable dye by following the protocol outlined below:
1)Gently vortex the ArC™ Amine Reactive Beads Kit and the AbC™ Anti-Mouse Bead Kit components for 30 seconds to completely resuspend before use.
2)Label a sample tube for the amine-reactive dye you are using, and add 1 drop of ArC™ reactive beads (Component A) to a labeled sample tube. Allow ArC™ reactive beads to sit in the tube for 5 minutes to warm to room temperature.
3)Prepare fluorescent reactive dye according to kit instructions included in the LIVE/DEAD® Fixable Dead Cell Stain Kit. For optimal performance of ArC™ reactive beads, always use freshly prepared amine-reactive dye. Do not use previously frozen dye solution.
4)Add the amount of LIVE/DEAD® fixable dead cell stain listed in Table 2 to the bead suspension and mix well. Make sure to deposit the amine-reactive dye directly to the bead suspension.
Table 2. Amount of amine-reactive LIVE/DEAD® fixable dead cell stain for use with ArC™ reactive beads.
Amine-reactive dye for use with ArC™ reactive beads | Amount |
LIVE/DEAD® Fixable Blue stain | 3 μL |
LIVE/DEAD® Fixable Violet stain | 1 μL |
LIVE/DEAD® Fixable Aqua stain | 3 μL |
LIVE/DEAD® Fixable Yellow stain | 3 μL |
LIVE/DEAD® Fixable Green stain | 3 μL |
LIVE/DEAD® Fixable Red stain | 1 μL |
LIVE/DEAD® Fixable Far Red stain | 3 μL |
LIVE/DEAD® Fixable Near-IR stain | 1 μL |
5)Label another sample tube for each fluorochrome-conjugated antibody you are using, and add 1 drop of AbC™ capture beads (Component A in the AbC™ Anti-Mouse Bead Kit) to each tube.
6)Add a pre-titrated amount of antibody conjugate to the appropriate tube and mix well. Make sure to deposit the antibody directly to the bead suspension.
7)Incubate for 30 minutes at room temperature, protected from light.
8)Add 3 mL of PBS or other buffer to each sample tube. Centrifuge at 300 × g for 5 minutes to collect the beads.
9)Carefully remove all supernatant from each tube. If using the red fluorescent reactive dye (Cat. no. L23102), repeat step 8 for that tube.
10)Resuspend the bead pellet by adding 0.5 mL of buffer to each sample tube.
11)Add one drop of negative beads (Component B in the AbC™ Anti-Mouse Bead Kit) to sample tube(s) containing the AbC™ capture beads.
12)Add one drop of ArC™ negative beads (Component B in the ArC™ Amine Reactive Compensation Bead Kit ) to sample tube(s) containing the ArC™ reactive beads.
13)Vortex tubes before analyzing using flow cytometry.
14)Perform manual or automatic compensation according to the preferred procedure for the flow cytometer in use. Gate on the bead singlet population based on FSC and SSC characteristics.





















