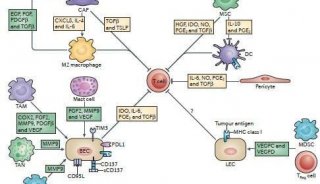
Xenograft Tumor Model Protocol
Preparation of tumor cells
Grow cells in complete medium and exclude any contamination
When cells are 70-80% confluent, 3-4 hrs before harvesting, replace medium with fresh medium to remove dead and detached cells.
Remove medium and wash cells with PBS. Add a minimum amount of trypsin-EDTA. Disperse cells and add complete medium (10:1 to 5:1). Centrifuge immediately at or below 1500 rpm for 2-5 min and wash twice with PBS and store cells on cell.
Count cells using a hemocytometer. Using trypan blue staining to exclude dead cells.
Mix cells 1:1 with trypan blue solution (Trypan Blue: dilute at 0.8 mM in PBS. Store at room temperature. Stable for 1 month.). Viable cells exclude trypan blue, while dead cells stain blue due to trypan blue uptake.
Cells should be suspended in a volume so that 300 µl contains required number of cells per injection. Usually, 3.0 x 106 cells are needed per injection.
Preparation of mice
Mice should be 4-6 weeks old.
Allow 3-5 days acclimatization period after mice have arrived.
Preparation of the injection
Clean and sterilize the inoculation area of the mice with ethanol and/or iodine solutions
Use 1-cc syringe and a 27- or 30-gauge needle
Mix cells and draw the cells into a syringe without a needle. Using a needle causes a strong, negative pressure which can cause cell damage and lysis.
Inject cells (3.0 x 106) subcutaneously (s.c.) into the lower flank of the mice.
Therapy can be started after 1-3 weeks when the tumors have reached an average volume of ~50–60 mm3.
Tumor diameters are measured with digital calipers, and the tumor volume in mm3 is calculated by the formula:
Volume = (width)2 x length/2