分子克隆-蛋白表达实验指南(十三)
7.
电泳结束后,按比例从胶上割下相应约1cm条带(当按比例的条带割下后可相应的向两边再割一点,但是电透析时中间和两边的胶必须分开透析),用镊子或尺子将胶碾碎成2mm见方的小块。
8. 将碎块小心加入电透析tube中,200V,120~150min。
9. 移出放电透析tube的架子,用注射器将tube上部的buffer吸掉。之后小心的在下部半透膜上插入注射器针头,缓缓析出其中的蛋白溶液。
10.吸取的蛋白溶液转入1.5ml EP管中,取20ul用于SDS-PAGE纯度验证,其余的-20C保存。
割胶的比例计算:
小胶(染色后): 大胶(未染色):
分离胶顶部――――― 分离胶顶部―――――
A cm
目的蛋白位置―――― B cm C cm 目的蛋白位置?
溴芬蓝底部位置――― 溴芬蓝底部位置――――
简单的比例计算即可得到大胶上目的蛋白的位置。
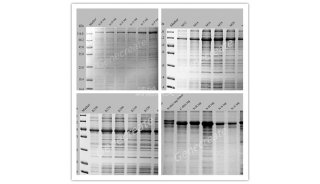
20.纯化后蛋白SDS-PAGE验证纯度
SDS-PAGE方法如前所述,该结果必须拍照存档, 格式如下:
Lane1~5:Marker、未诱导、诱导后、上清、沉淀
Lane6~9:纯化后蛋白依次按1.5, 0.75, 0.375, 0.187mg/ml上样,10ul each lane.
当lane 8看不到杂带时,蛋白纯度才符合要求
21. 其他常用操作及知识
SDS-PAGE:
蛋白质如果能融解在SDS溶液中,则它们可以按分子质量的大小加以分离。这种方法的原理为小分子质量的SDS与蛋白质的疏水基团结合,破坏了蛋白质的层叠结构,使它以舒展态稳定地存在于溶液中,每克蛋白质约结合1.4gSDS。SDS分子以它自己地负电荷掩盖了蛋白质分子原来存在的电荷,各种蛋白质分子表现出的电荷密度相等,SDS-蛋白质复合物的长度与其分子质量成比例,电泳时,它们纯粹按照分子量的大小由凝胶的分子筛效应进行分离。
层析柱的再生
Regeneration of Ni-NTA beads(from QIAGEN):
Handling
Ni-NTA matrices are stable under a wide variety of conditions and need not be
refrigerated, except to inhibit growth of microorganisms for long-term
storage. After use they should be washed for 30 minutes with 0.5M NaOH. Ni-NTA
matrices should be stored in 30% ethanol to inhibit microbial growth. The
matrix can be stored for up to one week in any of the denaturing buffers.
Reuse of Ni-NTA Resin
The reuse of Ni-NTA resin depends on the nature of the sample and should only
be performed with identical recombinant proteins. Based on the experience of
Hoffmann-La Roche Ltd. (Basel, Switzerland), who have purified more than 100
different proteins on Ni-NTA resin, we recommend a maximum of 5 runs per
column.
If the Ni-NTA Agarose changes from light blue to brownish-gray, the following
regeneration procedure is recommended.
Procedure:
1. Wash the column with 2 volumes of Regeneration Buffer (6 M GuHCl, 0.2 M
acetic acid).
2. Wash the column with 5 volumes of H2O.
3. Wash the column with 3 volumes of 2% SDS.
4. Wash the column with 1 volume of 25% EtOH.
5. Wash the column with 1 volume of 50% EtOH.
6. Wash the column with 1 volume of 75% EtOH.
7. Wash the column with 5 volumes of 100% EtOH.
8. Wash the column with 1 volume of 75% EtOH.
9. Wash the column with 1 volume of 50% EtOH.
10. Wash the column with 1 volume of 25% EtOH.
11. Wash the column with 1 volume of H2O.
12. Wash the column with 5 volumes of 100 mM EDTA, pH 8.0.
13. Wash the column with H2O.
14. Recharge the column with 2 volumes of 100 mM NiSO4.
15. Wash the column with 2 volumes of H2O.
16. Wash the column with 2 volumes of Regeneration Buffer.
17. Equilibrate with 2 volumes of a suitable buffer (e.g., Buffer A or B).
Regeneration of Glutathione Sepharose 4B(GST)
Glutathione Sepharose 4B may be regenerated for re-use by washing the gel with
2-3 bed volumes(Bed volume is equal to 0.5 x the volume of the 50% Glutathione
Sepharose slurry used or 0.75 x the volume of the originalGlutathione
Sepharose slurry) of alternating high pH (0.1 M Tris-HCl + 0.5 M NaCl, pH 8.5)
and low pH(0.1 M sodium acetate + 0.5 M NaCl, pH 4.5) buffers. This cycle
should be repeated 3 times followed by re-equilibration with 3-5 bed volumes
of 1X PBS.
If the gel appears to be losing binding capacity, it may be due to an
accumulation of precipitated, denatured or non-specifically bound proteins.
To remove precipitated or denatured substances, wash the matrix with 2 bed
volumes of 6 M guanidine hydrochloride, immediately followed by a wash with 5
bed volumes of 1X PBS.
To remove hydrophobically bound substances, wash the matrix with 3-4 bed
volumes of 70% ethanol or with 2 bed volumes of a non-ionic detergent (conc.
0.1%), immediately followed by a wash with 5 bed volumes of 1X PBS.
For long-term storage (>1 month), the following procedure of additional washes
is recommended:
1. Wash the gel twice with 10 bed volumes of 1X PBS.
2. Repeat washes using 20% ethanol.
3. Store at +4°C.
4. Re-equilibrate the gel with 1X PBS before re-use.
-
仪器推荐

-
仪器推荐
-
仪器推荐
 询底价 Tel:400-6699-117 转 5085
询底价 Tel:400-6699-117 转 5085 -
仪器推荐
-
仪器推荐