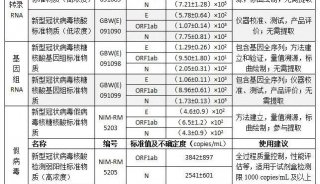
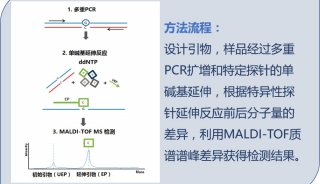
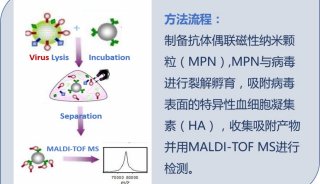
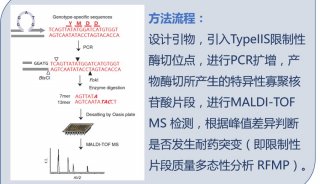
病毒核酸检测试剂盒英文使用说明
Lot-No.
Ref. FR340 Expiry time: 1 year
100 Tests (Ready to use PCR kit)
Zika Virus (Real time) DOUBLE CHECK
Only for in vitro use -
only for research use
-To be used by a technical person-
Principle and use
This amplification kit has been manufactured by Genekam Biotechnology AG, Germany to detect Zika-Virus (Double check. This is an absolute quantification assay. It tests twice the samples.
Real time PCR is based on fluorogenic dyes. In our kit we use 2 dyes
(2 probes), first Probe they are 6-Carboxy tetramethyl rhodamine
(quencher) and Carboxy-fluorescein (reporter; called also FAM-Channel).
Second Probe is labelled with HEX reporter (Filter for is VIC or in some
machines, it is HEX)on one side and on the other side 6-Carboxy
tetramethyl rhodamine (quencher). Up to 40 Ct for each dye should be
taken positive. Value between 40-45 Ct for each dye should be taken as
marginal positive (doubtful).
This kit needs RNA which can be
isolated from blood, serum, oral samples, liver samples, urine samples,
spleen, brain tissue, vaccine, seminal samples, lymph nodes, cell
cultures, tissue and any body fluid. Kindly use good methods to isolate
the RNA. Kindly take common safety laboratory precautions during working
as Zika virus is highly infectious. Please use gloves during work and
masks. Proceed clean and carefully otherwise you may cause contamination
problems. Do not touch other objects like pens, chairs etc. during Part
1.
IMPORTANT: we added cotton or sponge in the
lid of container of the kit, to avoid damage during transportation.
Please remove this cotton or sponge from the lid of each container
before storage.
Composition:
It contains the following: (Warning: THAW THE TUBES SLOWLY: NEVER THAW IN HEATING BLOCK OR WITH HEAT FROM HAND):
• Tube A (2 tubes)
• Tube B (2 tubes)
• Tube Y (1 tube)
• Positive (+Ve) control (D1): to be stored at -20°C (1 tube): It must be used with pipette tips with filter or it should be used with separate pipettor.
• Negative (-Ve) Control (tube D2) (1 tube)
Please check them before you start. Please keep all tube away from light.
Equipments needed:
• Realtime PCR thermocycler
• Laboratory centrifuge
• microtubes (0.2ml)
• sterile Pipette-tips with and without filter (20µl, 5µl & 1µl)
• Pipettes (quality pipettes)
• Paper
• Pen
• Microtube
• Ice
• Vortexer
• 96 well PCR plate
Procedure:
ONCE AGAIN:
VERY IMPORTANT ! PLEASE USE GLOVES ! DON’T TOUCH ANY OTHER OBJECTS, OTHERWISE THERE MAY BE RNASE CONTERMINATION DURING THIS PART.
STEP A
1. Kindly thaw one tube of: A, B, Y, D1 and D2. After thawing, kindly put the tubes at 4°C (as it is better). If the kit is not in use, store them at -20°C.
2. Mark your microtubes with a sample number, +ve Control and –ve Control. You can use 96 well microplate instead of tubes.
3. Add 7µl of tube A to each tube.
4. Add 10µl of B to each micro tube. Avoid to touch the wall of the microtubes.
5. Add 1µl of Y to each tube (avoid to touch the wall of the microtubes).
TIP: Add 7µl A + 10µl B + 1µl Y = 18µl per reaction. In case you want to run 10 reactions i.e. you need total 180µl, therefore you should mix 70µl of A + 100µl of B + 10µl of Y = 180µl from which you can take 18µl and add to each tube. This way you save time and hardware.
6. Add 2µl of your RNA with sterile pipette-tip with filter to each micro tube according to your label except +Ve and -Ve (Avoid touching the wall). Use every time a new pipette tip (for each sample) !
7. Use new pipette tip with filter. Add 2µl of tube D1. This is the positive control supplied with our kit. Mix it.
8. Use a new pipette tip. Add 2µl of –Ve (tube D2) to –Ve Control (don’t touch the wall).Mix it.
9. Centrifuge all tubes for 20 sec. for 8000 rpm (this is not necessary but it is better).
10.
Run the program of your thermocycler as followings: Kindly check
whether you have added everything correctly as the level of the volume
of each micro tube must be almost the same.
You must use quencher and reporter dye to setup your software (see FAQ) and run the following program:
1. 60 minutes at 42°C
10 minutes at 70°C
2. 15 seconds at 95°C x 45 cycles
60 seconds at 60°C
Before you start the PCR program, kindly check whether tubes are closed properly. Microtubes must be in contact with metal block (important!). There should be no air or lose contact with metal block of thermocycler.
11. After step 10 is finished take out the microtubes.
STEP B
Once the program will be finished one can see the graphics. The negative control should run along with the bottom and positive control must give a curve in the software graphics. Use your software to analyse the results.
Results: Usually there will be samples with two curves i.e. they are positive. There will be samples with one curve i.e they are positive. Samples without any curve are negative. The samples with two curves should have two Ct values i.e. they are double positive. The samples with one curve has only one Ct value i.e. positive samples. The samples without any curve will have no Ct values i.e. they are negative.
If you should find any mistakes, please let us know. Thank you.
Suggestion:
Last update: 15--12-2016 v1.0 | Genekam Biotechnology AG Germany Tel. (+49) 203 / 555858-31,-32,-33 Fax (+49) 203 / 35 82 99 http://www.genekam.de |
FAQ:
1) Q: I cannot find quencher and reporter dye in my software:
A: Many software has got the words: FAM (as reporter) and TAM (as quencher). Therefore select both in your software.
If your machines has only one word (for some machines only use the word FAM) you should select this one. For HEX, you can use HEX or VIC or JOE in your Software.
2) Precautions during the pipetting: Positive control should be pipetted with a separate pipettor and it should not be used for pipetting unknown samples or one should use pipette tips with filte
-
焦点事件

-
焦点事件

-
焦点事件

-
科技前沿

-
焦点事件

-
焦点事件

-
招标采购

-
焦点事件

-
焦点事件

-
焦点事件

-
企业风采

-
产品技术
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
政策法规

-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件

-
标准

-
焦点事件
-
政策法规
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
投融资

-
焦点事件

-
焦点事件
-
科技前沿

-
焦点事件

-
政策法规

-
焦点事件

-
招标采购

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
企业风采
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件

-
项目成果
-
市场商机
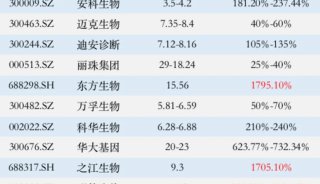
-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
科技前沿
-
招标采购
-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
项目成果
-
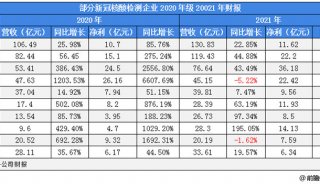
财报

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
标准
-
焦点事件

-
项目成果
-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件
-
项目成果

-
焦点事件

-
焦点事件
-
焦点事件
-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件

-
焦点事件
-
企业风采

-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件
-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
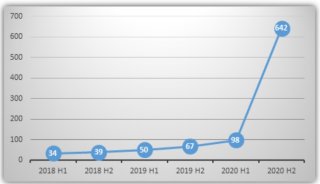
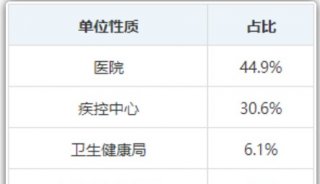
实验室动态

-
焦点事件

-
焦点事件
-
招标采购

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
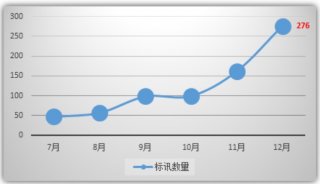
实验室动态

-
招标采购

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
项目成果

-
焦点事件

-
焦点事件

-
焦点事件

-
会议会展

-
企业风采

-
企业风采

-
焦点事件

-
焦点事件

-
企业风采

-
焦点事件

-
企业风采
-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
项目成果

-
项目成果

-
项目成果
-
项目成果
-
项目成果

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件

-
焦点事件
-
焦点事件

-
焦点事件

-
焦点事件

-
项目成果
-
焦点事件

-
焦点事件